Abstract
Elements with insulator/border activity have been characterized most extensively in Drosophila melanogaster. In vertebrates, the first example of such an element was provided by a hypersensitive site of the chicken β-globin locus, cHS4. It has been proposed that the homologous site in humans, HS5, functions as a border of the human β-globin locus. Here, we have characterized HS5 of the human β-globin locus control region. We have examined its tissue-specificity and assessed its insulating properties in transgenic mice using a lacZ reporter assay. Most importantly, we have tested its enhancer blocking activity in the context of the full β-globin locus. Our results show that HS5 is erythroid-specific rather than ubiquitous in human tissues. Furthermore, HS5 does not fulfil the criteria of a general in vivo insulator in the transgene protection assay. Finally, a HS5 conditional deletion from the complete locus demonstrates that HS5 has no discernable activity in adult erythroid cells. Sur prisingly, HS5 functions as an enhancer blocker in embryonic erythroid cells. We conclude that HS5 is a developmental stage-specific border in erythroid cells.
Keywords: border/globin/HS5/insulator/LCR
Introduction
The β-globin locus control region (LCR) drives high level, tissue-specific, copy number-dependent and position-independent expression of a linked transgene in mice (Grosveld et al., 1987). The key elements in the LCR are the five hypersensitive sites, named HS1 to 5, localized 6–22 kb (Tuan and London, 1984; Forrester et al., 1987) upstream of the ε-globin gene. Human β-thalassemia patients and transgenic mice with single or multiple deletions of these hypersensitive sites show a reduction in globin expression, rendering the transgenes susceptible to position effects (Milot et al., 1996; Bungert et al., 1999). Large deletions in a Dutch and a Hispanic thalassemia patient, removing HS1–5 and HS2–5 and upstream sequences respectively, resulted in a nuclease-resistant β-globin locus (Kioussis et al., 1983; Forrester et al., 1990). Other elements may cooperate or replace the chromatin activation function of the LCR, as deletion of the hypersensitive sites of the murine β-globin LCR does not affect the active chromatin structure in the β-globin locus (Epner et al., 1998; Reik et al., 1998; Bender et al., 2000; Tolhuis et al., 2002). Each hypersensitive site in the human LCR has different gene and stage-specific enhancing activities and they cannot functionally be replaced by each other (Fraser et al., 1993; Bungert et al., 1999), or by viral enhancer elements (Tanimoto et al., 1999b).
Activation of the β-globin locus is thought to be a multi-step process involving the recruitment of erythroid-specific transcription and chromatin remodeling factors (Levings and Bungert, 2002), resulting in a LCR holocomplex that directly interacts with the transcribed genes (Wijgerde et al., 1995; Carter et al., 2002; Tolhuis et al., 2002). The rate of transcription depends on the proximity of the genes to the LCR relative to the other genes (Hanscombe et al., 1991; Peterson and Stamatoyannopoulos, 1993; Dillon et al., 1997). The mode of action of the LCR to the globin genes appears to be orientation-dependent as an inverted LCR is incapable of activating downstream globin genes at high level, and the LCR fails to activate an ε-globin gene inserted upstream of LCR (Tanimoto et al., 1999a). Thus, the LCR may be intrinsically unidirectional, e.g. through the spatial arrangement of hypersensitive sites, or that one of the elements blocks LCR action in one of the directions.
Elements that specifically promote or inhibit interactions have been characterized extensively in Drosophila. These include the SCS elements located around the hsp70 gene (Kellum and Schedl, 1991), the gypsy transposable elements (Roseman et al., 1993) and the Fab fragments (Fab7 and Fab8) in the BX-C complex that insulate interactions between various iab 6–8 tissue-specific enhancers (Zhou et al., 1996). In vertebrates such elements were first reported to be present in the chicken β-globin locus. 5′-HS4 (cHS4) in the chicken β-globin locus marks the border between the active chromatin structure of the β-globin locus and the upstream 16 kb low level of histone acetylation, fully methylated and condensed nuclease-insensitive heterochromatic region (Hebbes et al., 1994; Prioleau et al., 1999; Litt et al., 2001a,b). The element was tested for both enhancer blocking activity and transgene protection or barrier properties. cHS4 blocks the interaction between an enhancer and promoter on a plasmid, thereby reducing reporter gene expression in cell lines and protecting the transgene from position effects in Drosophila (Chung et al., 1993, 1997; Recillas-Targa et al., 2002). cHS4 also act as a barrier to alleviate the silencing effects (Pikaart et al., 1998; Emery et al., 2000, 2002). The 11 Zn-finger protein, CTCF, which binds to the FII region within cHS4, is responsible for the enhancer blocking activity, but is separable from the insulating activity of cHS4 (Burgess-Beusse et al., 2002; Recillas-Targa et al., 2002).
HS5 is the homologue of the cHS4 in the human β-globin locus (Hardison et al., 1997; Li et al., 1999). Unlike in the chicken β-globin locus, the human β-globin locus is not flanked on the 5′ side by a repetitive heterochromatic region, but has an open chromatin structure. Similar to cHS4, it contains CTCF binding sites (Farrell et al., 2002) and has been reported to be a ubiquitous hypersensitive site as it is present in several cell lines (Tuan et al., 1985). Human HS5 is believed to be an in vivo insulator because it possesses both enhancer blocking and transgene protection activities (Li and Stamatoyannopoulos, 1994; Li et al., 1999; Farrell et al., 2002). Deletion of HS5 shows no apparent effect on globin gene transcription, nor does it show any enhancing activity when linked to a reporter gene construct (Reik et al., 1998; Li et al., 2002). This suggests that HS5 may be involved in providing polarity to the action of the LCR. However, HS5 has never been tested in its natural configuration as part of the full β-globin locus. In this study, we report on the structural and functional properties of HS5 in vivo.
Results
Erythroid specificity and mapping of HS5 of the human LCR
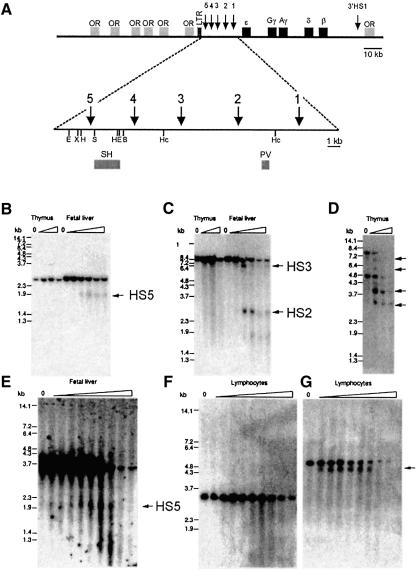
We first determined the position of HS5 using a single-copy transgenic line carrying the human β-globin locus (Figure 1A; Strouboulis et al., 1992). Fetal liver, thymus and brain were used for DNase I hypersensitive site mapping. HS5, like HS2 and HS3, was absent from mouse non-erythroid cells but present in erythroid fetal liver cells (Figure 1B and C). The presence of a hypersensitive site in the murine vav gene in the thymus series (Figure 1D; Ogilvy et al., 1998) demonstrates that the absence of detectable globin LCR hypersensitive sites was not due to technical problems. We confirmed the erythroid specificity of HS5 through the analysis of chromatin structure in human tissues. The hypersensitive fragment (2.0 kb) for HS5 was clearly detected in human fetal liver (Figure 1E) but was absent from peripheral blood lymphocytes (Figure 1F). The quality of the lymphocyte fade-out was checked by probing the same filter with a human CD2 probe detecting the 3′-HS (Greaves et al., 1989; arrow in Figure 1G). We therefore conclude that HS5 of the LCR is an erythroid-specific hypersensitive site.
Fig. 1. HS5 of the human β-globin locus is erythroid-specific. (A) The top line shows the human β-globin locus. The five globin genes and the LTR element are indicated. Arrows show hypersensitive sites. E, EcoRI; X, XbaI; H, HindIII; B, BamHI; Hc, HincII; S, SacI. Probes used: PV, 450 bp PvuII–EcoR fragment and SH, 1.3 kb SacI–HindIII fragment. (B–G) In vivo DNase I hypersensitive site mapping. Nuclei were prepared from E13.5 fetal livers and young animals (thymus) of the β-locus line 72 (B, C, D); human fetal liver at 16 weeks of gestation (E) and adult peripheral blood (F and G), and digested with increasing amounts of DNase I. DNA was digested with HindIII (B), HincII (C), BglII (D), BamHI–EcoRI (E) and BamHI–XbaI (F and G), Southern blotted and probed with SH (B, E, F) and PV (C). The wedge above each panel indicates increasing amounts of DNase I. ‘0’ indicates no DNase I. Arrows indicate DNase I hypersensitive sites. As a control for hypersensitivity, the thymus DNase I series in (B and C) were used in (D) and probed with a 950 bp NcoI fragment of the murine vav gene. A duplicate filter of (F) was used in (G) and probed with a 600 bp SacI–HindIII fragment of the human CD2 gene to detect the 3′ hypersensitive site.
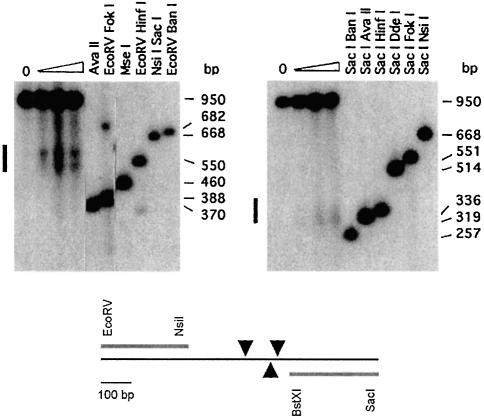
Next, the position of HS5 was mapped in detail using nuclei prepared from a murine erythroleukemia (MEL) cell clone stably transfected with three copies of the minilocus ε-globin construct (Lindenbaum and Grosveld, 1990; Figure 2). HS5 mapped to an ∼200 bp core fragment, marked in Figure 1A. We performed in vitro DNase I footprinting on a 270 bp fragment encompassing the HS5 core sequence (Supplementary figure 1, available at The EMBO Journal Online). Several footprints and hypersensitive sites were found throughout the fragment in fetal liver, MEL cell, adult spleen and adult liver nuclear extracts, including the CTCF binding site (Farrell et al., 2002). When the individual footprints were investigated by band shift analysis, only very weak bands were detected (not shown). This is in sharp contrast to the strong shifts observed with control probes derived from HS3 (Philipsen et al., 1993). These results precluded the further analysis of the proteins interacting with HS5. We therefore proceeded to determine whether HS5 activates a transgene in vivo and/or whether it has enhancer blocking/insulating properties in transgenic mice.
Fig. 2. DNase I fine mapping of HS5 in vivo. Nuclei from MEL cells with three copies of a cosmid containing the human β-globin LCR and ε-globin gene were digested with increasing amounts of DNase I. DNA was purified, cut with SacI and EcoRV, and Southern blotted. Internal molecular weight markers were obtained by cutting DNA from the ‘no DNase I’ sample (0) with the restriction enzymes indicated. Probes used are a 271 bp EcoRV–NsiI fragment (5′ probe; left panel) and a 300 bp BstXI–SacI fragment (3′ probe, right panel). The location of major DNase I cleavage sites (arrowheads) and the positions of the probes is shown below.
Gene regulatory properties of HS5 assayed in transgenic mice
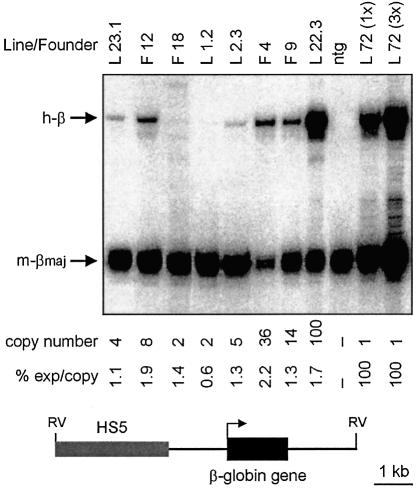
HS2 and HS3 of the β-globin LCR confer high-level expression to a linked β-globin gene in transgenic mice (Philipsen et al., 1990; Talbot et al., 1990). To determine whether the human HS5 has any gene activation activity, a 3.0 kb fragment encompassing HS5 was cloned 5′ of a β-globin gene and injected as a 7.5 kb EcoRV fragment to generate transgenic mice (Figure 3). Expression levels were analyzed by S1 nuclease protection of probes specific for the 5′ ends of the human and mouse β-globin mRNAs (Figure 3). RNA was obtained from E13.5 fetal livers of four founders and four bred lines. E13.5 fetal liver RNA from mice carrying the 70 kb human globin locus was used as control (Strouboulis et al., 1992). In all mice, the expression per copy of the human β-globin mRNA was very low (0.6–2.2% of the murine β-globin; Figure 3). These levels are comparable to those of transgenic mice containing the β-globin gene without a linked LCR fragment (Philipsen et al., 1993). The variability of the level of expression between transgenic lines may be slightly decreased (1.44 ± 0.49) when compared with the expression of observed previously with the β-globin gene alone (1.0 ± 0.8). Expression of the β-globin gene alone was not detectable in two out of six independent transgenic fetuses analyzed (a 2- and an 8-copy animal). In contrast, all eight multi-copy HS5–β-globin transgenics express, including the 2-copy animals. This suggests HS5 protects the transgene from the repressive action of chromatin at the integration site, but that it is not a transcriptional activator in erythroid cells.
Fig. 3. Activity of HS5 in erythroid cells. Fetal liver RNA was isolated from E13.5 fetuses carrying the HS5–β-globin transgene (bottom line). Expression of the human β-globin transgene was analyzed by quantitative S1 nuclease analysis. Expression was calculated as (human β-globin signal/transgene copy number)/(mouse β-major signal/2) and was set at 100% for line 72. F, founder; L, line; ntg, non-transgenic; 72 (1×), line 72 E13.5 fetal liver RNA; 72 (3×), 3 × the amount of line 72 E13.5 fetal liver RNA to demonstrate probe excess.
Insulator properties of HS5 in transgenic mice
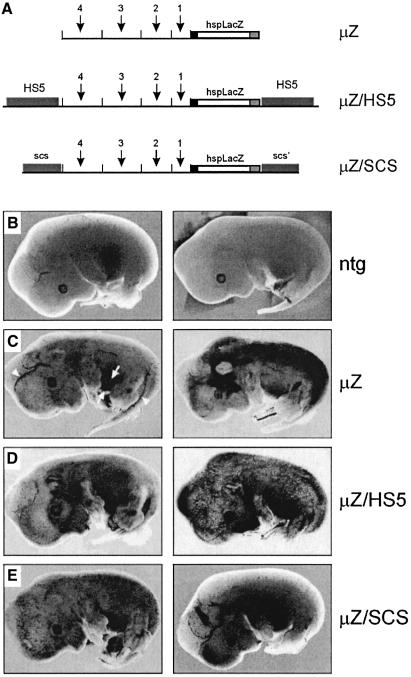
We set up transgenic experiments to test the possibility that HS5 functions as an insulator or acts to prevent integration site-dependent regulatory influences on a reporter gene. The strategy was similar to that of Kellum and Schedl (1992) who showed that the SCS elements from the Drosophila heat shock gene, hsp70, are capable of insulating a test gene from chromosomal position effects. We chose the β-galactosidase (LacZ) gene and the mouse hsp68 minimal promoter because this construct is highly susceptible to position effects (Kothary et al., 1989; Tewari et al., 1996). The expression of the transgene was brought under the control of the µLCR to direct expression to the erythroid lineage (µZ, Figure 4A; Tewari et al., 1996). In a second construct, µZ is flanked on both sides by the 3 kb fragment carrying HS5. If HS5 functions as an insulator, this transgene should express in the erythroid lineage but not in any other tissues, irrespective of the site of integration. As a second insulator plasmid, we tested the Drosophila SCS and SCS′ elements (Kellum and Schedl, 1992) flanking µZ (µZ/SCS). These constructs were introduced in fertilized eggs and the resulting fetuses assayed for β-galactosidase activity at E13.5. Examples are shown in Figure 4C–E.
Fig. 4. Position effect assay in transgenic mice. (A) Constructs µZ, µZ/HS5 and µZ/SCS contain the bacterial β-galactosidase gene driven by a 100 bp hsp68 promoter fragment (hspLacZ). The arrows and numbers indicate the individual hypersensitive sites in the µLCR construct. (B–E) Examples of transgenic fetuses (E13.5) stained for β-galactosidase activity. The transgene is indicated on the right; two different fetuses are shown for each construct.. Ntg, non-transgenic control fetuses. Erythroid tissues at this stage of development are indicated in panel C: the fetal liver (the site of erythropoiesis at the fetal stage) is in between two arrows; arrowheads point to the major vasculature containing blue-stained circulating erythrocytes. The presence of these cells in capillaries results in the blue-spotted appearance of the fetus.
Ten out of twelve µZ fetuses express in erythroid tissue (Figure 4C, left panel), but five of these ten fetuses also express in a wide range of tissues, e.g. the nervous system, limbs and snout (Figure 4C, right panel). The remaining two fetuses showed ectopic expression, but did not express in the erythroid tissue possibly due to mosaicism of the transgene (Grosveld et al., 1987). Of the seven transgenic µZ/HS5 fetuses, all expressed in erythroid tissue while three of these seven animals also showed expression in other tissues (Figure 4D). These results indicate that HS5 is not capable of insulating a reporter gene from positive position effects in non-erythroid tissues. Similarly the Drosophila SCS elements do not protect the µZ/SCS construct from position effects in transgenic mice (Figure 4E). We conclude that HS5 does not protect the hsp-LacZ transgene from position effects in non-erythroid cells, and is therefore not a ubiquitously active insulator. This is in contrast to data obtained from cell culture experiments, that were interpreted to demonstrate such insulator properties of human HS5 (Li and Stamatoyannopoulos, 1994; Li et al., 2002).
PAC constructs for functional analysis of HS5 in the context of the human β-globin locus
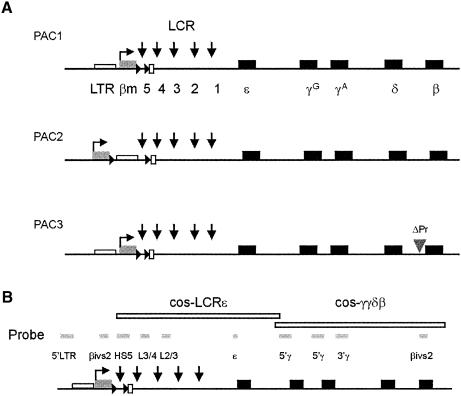
To examine the in vivo role of HS5 and LCR polarity in the context of the β-globin locus, we generated transgenic mice containing human β-globin locus constructs. These were made from a 185 kb human β-globin locus PAC clone by recA-mediated homologous recombination (Imam et al., 2000). To test for the directionality of the LCR and enhancer-blocking effect of HS5, we placed a marked β-globin (βm) gene upstream of HS5, in the same orientation as the normal β-globin (β) gene (Figure 5A). The βm gene contains internal γ-globin sequences that allow the βm transcripts to be distinguished from those generated by the normal γ- and β-globin genes (Dillon et al., 1997). We flanked HS5 with loxP sites to enable Cre-mediated deletion of HS5 (Figure 5A). A single FRT site was also inserted at the 3′ loxP site, to generate single-copy transgenic animals from multi-copy containing animals with Flp recombinase (Figure 5A; PAC1). To examine the effect of distance, and the potential role of an upstream long terminal repeat of an endogenous retrovirus (LTR element, Long et al., 1998), we also moved the βm gene and the 5′ loxP site upstream of HS5 and the LTR (Figure 5A, PAC2). In PAC1, competition between the two β-promoters for interaction with the LCR (Wijgerde et al., 1995) might obscure the interpretation of the results. To alleviate this problem, we deleted the promoter of the normal β gene in PAC1 by homologous recombination (Figure 5A, PAC3). The three constructs were carefully mapped on Southern blots. We used 11 different restriction enzymes and hybridization with cosmid LCRε and -γγδβ probes (Strouboulis et al., 1992) and smaller probes along the β-globin locus (Figure 5B), to ensure that the modifications were made in the designated positions and that there were no rearrangements in the modified PACs.
Fig. 5. Constructs for the generation of mutant β-globin PAC transgenic mice. (A) PAC1: HS5 is flanked by a βm gene, a loxP site (triangle) inserted upstream, and loxP (triangle)/FRT (open rectangle) sites inserted downstream of HS5. PAC2: same as PAC1 except the βm gene and 5′ loxP site are moved upstream to the LTR element. PAC3: same as PAC1 except with a deleted β gene promoter (ΔPr). (B) Probes used to characterize the PAC1, -2 and -3 plasmids, and transgenic mice. Map is not drawn to scale.
Transgenic mice were generated with the purified 185 kb PAC inserts into fertilized mouse oocytes, after removal of the 12 kb vector fragment. It is mandatory to study single-copy transgenic mice, as multi-copy inserts in tandem repeats result in interactions that normally do not take place in a single-copy locus. Unfortunately, the generation of single-copy animals from multi-copy transgenics through Flp-mediated recombination did not work, possibly due to the large distance between the FRT sites and the weak recombinase activity of Flp in mammalian cells (Ringrose et al., 1998). We therefore generated sufficient founder mice to select single-copy transgenics for analysis. The copy number of the transgene was determined by hybridizing Southern blots of BamHI-digested DNA with a human β-globin gene-specific probe together with an internal mouse Col10a1 probe (see Supplementary figure 2 and Kong et al., 1993). The results for all three constructs and the integration site of the single-copy transgenics are summarized in Table I.
Table I. Transgenesis results and integration sites of the mutant β-globin PAC constructs.
| Construct | Founders | Transmitted | Multiple copies | Single copy with an intact locus | Site of integration(single-copy line) |
|---|---|---|---|---|---|
| PAC1 | 6 | 6 | 1 | 2 | PAC1-A |
| Non-centromeric | |||||
| PAC1-B | |||||
| Centromeric | |||||
| PAC2 | 8 | 7 | 2 | 2 | PAC2-M |
| Non-centromeric | |||||
| PAC2-N | |||||
| Non-centromeric | |||||
| PAC3 | 18 | 10 | 5 | 3 | PAC3-G |
| Non-centromeric | |||||
| PAC3-H | |||||
| Non-centromeric | |||||
| PAC3-K | |||||
| Non-centromeric |
Analysis of PAC transgenic mice and Cre-mediated excision
Owing to the large size of the PAC constructs, transgenic lines might carry truncations, internal deletions and rearrangements in the transgenes. To ensure the integrity of the constructs in the single-copy transgenic lines, DNA of F1 offspring was carefully mapped with both cosmid probes and individual probes along the locus (Figure 5B). The combination of individual β-globin locus probes and three different enzyme digests detects large overlapping restriction fragments of the mutant β-globin locus on Southern blots. Transgenic lines showing unexpected hybridization patterns with any of the globin probes tested were discarded for further analysis (see Supplementary figure 2). This mapping scheme assures that the β-globin locus is intact in the single-copy transgenic lines from 14.3 kb upstream of HS5 to 8.2 kb downstream to the β-globin gene.
We used the single-copy PAC lines to delete HS5 (PAC1 and PAC3) or LTR + HS5 (PAC2) by crossing the PAC lines with Cre-expressing mice (Sakai and Miyazaki, 1997). We found that Cre-mediated deletion of HS5 or LTR+HS5 in the PAC transgenics was very efficient (Supplementary figure 2 and data not shown), resulting in the lines PACΔ1, PACΔ2 and PACΔ3.
Directional activation properties of the LCR
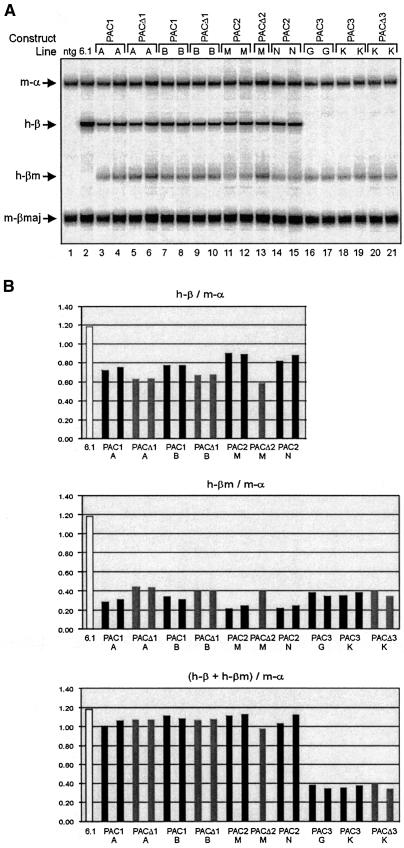
We used the six different PAC transgenes described above to test LCR directionality and HS5 enhancer-blocking activity in adult erythropoiesis. We determined the expression of the βm- and β gene by S1 nuclease protection (Antoniou, 1991; Dillon et al., 1997). In the adult blood of the PAC1 (Figure 6, line A, B) and PAC2 (line M, N) transgenics, both the upstream βm and downstream β gene are transcribed, indicating that the LCR can activate genes in an upstream and downstream direction. This is in apparent contrast to previous data, suggesting the LCR is unidirectional and unable to activate a mutated ε-globin gene placed upstream of HS5 (Tanimoto et al., 1999a). However, we note that the βm gene in the PAC1 and PAC2 transgenic mice is expressed at a relatively low level, when compared with the β gene (Figure 6). The βm gene is much closer to the LCR than the β gene and should therefore have a competitive advantage for transcriptional activation by the LCR (Hanscombe et al., 1991; Dillon et al., 1997) and hence our data provide evidence for a directional component in LCR function, in agreement with Tanimoto et al. (1999a). In further agreement with previous models proposed for LCR function (Wijgerde et al., 1995), expression of the βm gene is in competition with and therefore at the expense of the β gene (Figure 6B). The total transcriptional output of the PAC1 and PAC2 loci is unaltered relatively to the output of a wild-type control PAC.
Fig. 6. Expression analysis of the human β-globin PAC transgenes in adult transgenic mice. (A) Quantitative S1 protection assay was performed in adult blood samples to study the changes in level of βm- and β expression in the PAC1 (A and B); PAC2 (M and N) and PAC3 (G and K) lines before and after HS5 deletion (PACΔ1, PACΔ3), or 5′ LTR+HS5 deletion (PACΔ2). Control samples are from a wild-type β-globin PAC transgenic line (6.1); and a non-transgenic mouse (ntg). Arrows indicate the positions of protected fragments for mouse α-globin (m-α), mouse β-major globin (m-βmaj), human β-globin (h-β) and human βm globin (h-βm). (B) Quantitation of the expression of the βm- and β genes in adult transgenic mice. The bar graphs depict the levels of βm and β expression, relative to mouse α-globin expression and after correction for the specific activities of the S1 probes. Black bars: PAC1, -2 and -3; grey bars: PACΔ1, Δ2 and Δ3; white bars: control PAC, after correction for expression per copy.
The notion of directionality is most convincingly demonstrated by the analysis of PAC3, in which the promoter of the β gene is deleted. This resulted in a consistent but only marginal increase in the expression of the βm gene, compared with PAC1 and PAC2 (Figure 6). The presence of HS5 could be an important determinant of directionality (Tanimoto et al., 1999a). We therefore analyzed the expression of the βm- and β-gene in the PACΔ1, Δ2 and Δ3 lines. Expression of βm in lines PACΔ1 and Δ2 is modestly increased with by a comparable decrease in β expression. This increase in expression is most likely due to the shorter distance of the βm gene to the LCR after Cre-mediated excision, as the increase is more pronounced in the PACΔ2 lines where the gene is moved 3.6 kb closer to the LCR compared with 0.8 kb in the PACΔ1 lines. Most interestingly, expression of the βm gene is not altered in the PACΔ3 lines. Thus, in absence of competition from the β gene, moving the βm gene closer to the LCR does not result in increased expression. This is consistent with the notion that the βm gene competes with the β gene in the other PAC lines. We further conclude that HS5 and the LTR element do not possess enhancer-blocking activity in adult erythroid cells. Finally, the relatively inefficient expression of the βm gene, even in the absence of competition from the promoter of the β gene, supports the notion of directionality of the LCR. Our data demonstrate that HS5 and the LTR element are dispensable for this property of the LCR.
HS5 function in primitive erythropoiesis
In mammals, the first wave of erythropoiesis occurs early during embryogenesis in the yolk sac, referred to as primitive erythropoiesis. In the mouse, this takes place between E8 and E11. It is distinct from fetal liver and adult bone marrow erythropoiesis, referred to as definitive erythropoiesis. Primitive cells express embryonic/fetal-type globins while definitive cells express fetal/adult-type globins. In transgenic mice carrying the human β-globin locus, primitive cells express the human ε- and γ-globin genes (Strouboulis et al., 1992). In the adult bone marrow, only the β-globin gene is expressed. The order of the genes, and distance to the LCR, are important parameters in this developmental switch (Hanscombe et al., 1991; Dillon et al., 1997). When the ε-globin gene is replaced by a βm gene, the βm gene is already highly transcribed in primitive cells and competes efficiently with the γ-globin genes for activation by the LCR (Dillon et al., 1997). In adult erythroid cells, the ε- and γ-globin promoters are repressed, thus allowing the activation of the β-globin gene which is most distant to the LCR. These developmental switches are accompanied by changes in chromatin structure throughout the locus (Gribnau et al., 2000). It is therefore of considerable interest to determine embryonic expression of the PAC transgenes generated in this study.
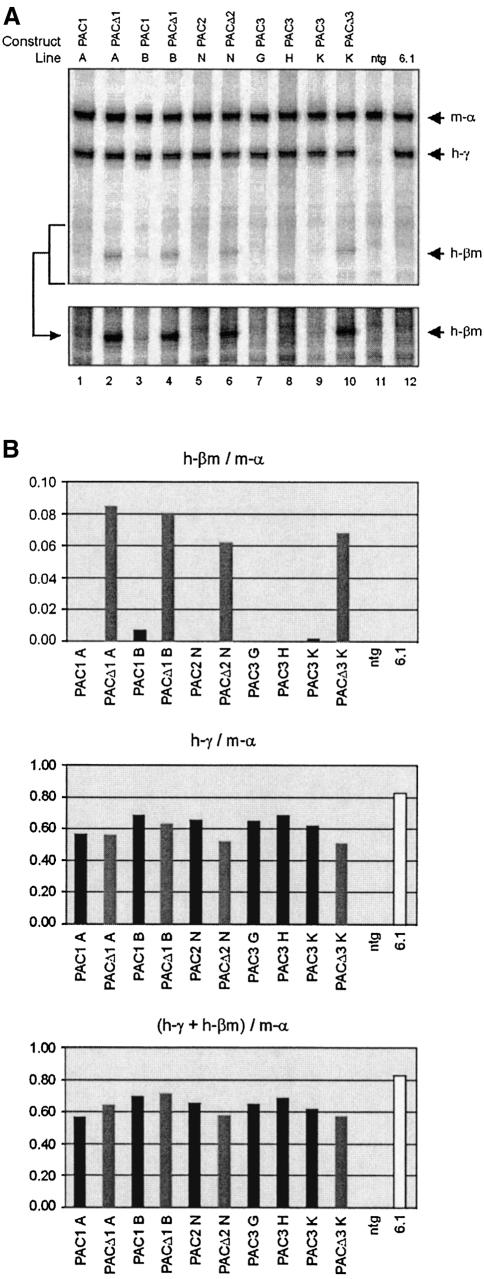
We collected E10.5 yolk sacs of the different PAC lines and determined the expression of the βm- and γ-globin genes by S1 nuclease protection. In this case, the S1 probe for γ-globin also detects βm transcripts and the signals can therefore be compared directly. As an internal control, we used a probe for mouse α-globin. To our surprise, and in sharp contrast with previous data obtained when the βm gene replaced the ε-globin gene (Dillon et al., 1997), we failed to detect appreciable expression of the βm gene in any of the PAC1, -2 and -3 lines (Figure 7). This suggests that activation of βm is blocked by an element with border properties, presumably HS5. To test this hypothesis, we analyzed expression of the βm- and γ-globin genes in the PACΔ1, Δ2 and Δ3 lines. We found considerable levels of expression of the βm gene in these lines (Figure 7). Because deletion of HS5 alone is sufficient for this effect, we conclude that HS5 has potent enhancer-blocking activity in primitive erythroid cells. As a consequence, the βm promoter can only interact productively with the LCR upon removal of HS5. In this situation the βm promoter may compete with the ε- and γ-globin promoters for activation by the LCR. However, we did not observe a significant reduction in γ-globin expression upon deletion of HS5 (Figure 7B). Since such competition would affect the efficiency of four promoters simultaneously, the overall effect on ε- and γ-globin transcription might be too modest to be clearly visible in the protection assay. Furthermore, activation of the βm gene in the upstream position is inefficient compared with that observed when the gene is in the position of the ε-globin gene (Dillon et al., 1997). In accordance with the data in adult cells, this suggests a directional component in LCR function in embryonic cells.
Fig. 7. Expression analysis of the human β-globin PAC transgenes in transgenic mouse embryos. (A) Quantitative S1 protection assay carried out on E10.5d.p.c. yolk sac samples of PAC1 (A and B); PAC2 (M and N) and PAC3 (G, H, K) lines before and after HS5 deletion (PACΔ1, PACΔ3), or 5′ LTR+HS5 deletion (PACΔ2), to determine the expression levels of βm- and γ-globin. A longer exposure of the lower part of the gel is shown at the bottom. (B) Quantitation of the expression of the βm- and γ-globin genes in transgenic mouse embryos. The bar graphs depict the levels of βm and γ-globin expression, relative to mouse α-globin expression and after correction for the specific activities of the S1 probes. See legend to Figure 6 for other details; the arrow with h-γ indicates the position of the protected fragment for γ-globin. The scale on the y-axis is changed in the top panel, to accommodate for the relatively low expression levels of the βm gene.
Discussion
HS5 is an erythroid-specific hypersensitive site
In this study, we have characterized the structural and functional properties of HS5 of the human β-globin LCR. DNase I hypersensitive site mapping shows that the HS5 core is an erythroid-specific hypersensitive site; this disagrees with the current consensus that human HS5 is a ubiquitous hypersensitive site (Tuan et al., 1985; Forrester et al., 1987). Unlike the previous studies that used immortalized cultured cells, we mapped HS5 specifically in erythroid and non-erythroid tissues from humans or mice carrying the human β-globin locus. Thus, the ubiquitous presence of human HS5 reported previously appears to be a consequence of the analysis of this site in cell lines. Furthermore, HS5 of the mouse globin locus is also not ubiquitously present (Li et al., 1999). Hence we conclude that HS5 is erythroid-specific.
Transcriptional activation activity of HS5 in erythroid cells
We followed several approaches to dissect the functional properties of HS5 in vivo. Firstly, we found that HS5 does not possess enhancer properties when linked to a β-globin reporter gene in transgenic mice. This reporter gene was expressed at levels similar to those observed with the β-globin gene alone. This is consistent with recent data on mouse HS5, which also failed to demonstrate transcriptional activation properties of HS5 in transgenic mice (Li et al., 2002).
HS5 is not a general insulator element
The high-level, position-independent expression observed with full LCR/globin constructs can be explained by two non-mutually exclusive mechanisms. The LCR may act as a dominant regulator overriding any integration site-dependent influences and/or the LCR may insulate the transgene from such position effects (Grosveld et al., 1987). The homologue of HS5 in the chicken β-globin locus, cHS4, marks the boundary between active and inactive chromatin (Hebbes et al., 1994; Prioleau et al., 1999). Inversion of the full human LCR significantly reduces the expression level of all the genes in the locus, and the LCR fails to activate an ε-globin gene inserted upstream of HS5 (Tanimoto et al., 1999a). These observations suggest that HS5 could function as an insulator element in the human β-globin locus. We therefore tested potential insulator properties of HS5 in transgenic mice. We flanked the reporter gene either by HS5 (µZ/HS5) or by known insulator elements from Drosophila, the scs elements (µZ/SCS). Surprisingly, neither HS5 nor the scs elements are able to protect the reporter gene from position effects in transgenic mice. This would appear to contradict previous reports on cHS4 and human HS5 (Chung et al., 1993; Li et al., 2002), but this discrepancy could well be explained by the different assay systems used. Chung et al. used cultured cells and Li et al. analyzed HS5 function in erythroid tissues only. It is interesting to note that the Drosophila scs elements do not appear to have any effect in the mouse with respect to position effects, whereas the chicken element was reported to be active in both mammalian cells and Drosophila (Chung et al., 1993). Based on the read-out of our lacZ position-effect assay, we conclude that HS5 does not function as a general insulator element.
Function of HS5 in the context of the human β-globin locus
The insulator properties of human HS5 were also assessed in the context of the whole β-globin locus. We characterized single-copy transgenic mice to ensure that the β-globin locus is intact and that there is no influence of globin cis-regulatory elements present in tandem transgene arrays. Conditional deletion of HS5 was carried out at fixed chromosomal sites in the transgenics. Therefore, any change in globin expression can only be attributed to the deletion. In PAC1 transgenic mice, a βm gene is inserted immediately upstream of HS5. Interestingly, this βm gene is expressed at substantially lower levels than the normal β gene located ∼65 kb downstream of HS5. Competition with the normal β-gene for activation by the LCR is inefficient when the βm gene is situated upstream from the LCR, in comparison with that observed when a βm gene is placed immediately downstream of the LCR. In the latter case, expression of the βm gene is dominant over the normal β gene (Dillon et al., 1997; Tanimoto et al., 1999a). There are two, not mutually exclusive, explanations for this phenomenon. Firstly, HS5 might have enhancer-blocking properties as has been shown by in vitro assays (Farrell et al., 2002). Secondly, the activation potential of the LCR might be different for genes lying upstream or downstream of the LCR. Since deletion of HS5 in the PAC1 and PAC2 results in only a slight increase (31 and 39% respectively) in the level of βm expression, we conclude that HS5 does not play a significant role in blocking the action of the LCR on the upstream βm gene in adult erythroid cells. This increase in βm expression is readily explained by the fact that the βm gene is moved closer to the LCR by the Cre-mediated deletions. This notion is supported by the more pronounced effect observed in the PAC2 lines, in which the βm gene is positioned further upstream from the LCR than in PAC1 before Cre-mediated deletion, but in exactly the same position after excision in PACΔ1 and PACΔ2. Importantly, expression levels of the βm gene are identical in the PACΔ1 and PACΔ2 lines. This indicates that the LTR, still present in PACΔ1 but absent in PACΔ2, has no influence on the βm gene or the LCR. The situation is very different in primitive erythroid cells. In these cells, the βm gene is not expressed at appreciable levels in the PAC1, -2 and -3 lines. We have previously demonstrated that the βm gene is expressed at high levels at this stage when placed immediately downstream of the LCR (Dillon et al., 1997). When HS5 is deleted, either with or without the LTR, to result in PACΔ1, Δ2 and Δ3, the βm gene is active. Thus, we conclude that HS5 is a developmental-specific border in the context of the human β-globin locus. Genetic elements conferring directionality and tissue-specific blocking activity in mammalian gene loci have been described previously, e.g. in the Hox gene cluster (Kmita et al., 2002). To the best of our knowledge, mammalian border elements with developmental specificity have not been reported before. HS5 of the human β-globin LCR therefore provides the first example of a genetic element with these properties.
HS5: a border without a cause?
The most interesting observation reported here is that HS5 acts as a developmental-specific border in erythroid cells. This begs the question why there would be a requirement for this property of HS5. It is possible that such a requirement no longer exists. In this scenario, HS5 might be an evolutionary remnant of an earlier globin locus in which this activity was required. Dillon and Sabbattini (2000) have proposed that elements interfering with enhancer/promoter interactions are actively selected against within gene clusters. As a result, elements with such interfering activities would preferentially be found near the borders of expression domains, without being positively selected as a boundary element. Alternatively, this property of HS5 might reflect fundamental differences between embryonic and adult chromatin. It has been suggested previously that embryonic chromatin is much more permissive for transcription than adult chromatin (Tewari et al., 1996; Wolffe, 1996). Thus, the border properties of HS5 might prevent interactions between the LCR and as yet unknown upstream promoter elements in embryonic cells, as we observe with the βm gene when HS5 is deleted. Such interactions would normally be unproductive, and therefore even a small contribution of the border function to the efficiency of the activation of the embryonic globin genes might be positively selected for. In adult cells, the non-permissive nature of chromatin would be sufficient to prevent these undesired interactions, and hence the border function of HS5 has become redundant. In this regard, it is interesting to note that expression of the adult β-globin gene is entirely dependent on the presence of the transcription factor EKLF (Wijgerde et al., 1996) and that EKLF remodels the chromatin structure of the LCR (Gillemans et al., 1998). In contrast, the embryonic/fetal ε- and γ-globin genes are expressed at normal levels in EKLF null cells. This observation is particularly striking because EKLF is known to be present in wild-type embryonic erythroid cells (Southwood et al., 1996) and to function as a transcriptional activator in these cells (Tewari et al., 1998). It will therefore be of great interest to determine the expression pattern of the βm gene in the PAC1, -2 and -3 lines in an EKLF null background, and to evaluate the border function of HS5 in the PACΔ1, Δ2 and Δ3 in this context. These experiments are in progress.
Materials and methods
Hypersensitive site mapping
Human lymphocytes were obtained from blood following standard procedures. Nuclei from mouse, human tissues and cultured cells were prepared as described (Forrester et al., 1990). DNase I concentrations ranged from 0 to 0.24 µg/ml. Digestions were carried out at 37°C for 4–8 min. After purification, the samples were analyzed by Southern blotting.
Generation and analysis of constructs and mice
A 7.5 kb EcoRV fragment containing the HS5 (3 kb BamHI–EcoRI from cos-LCRε (Figure 5B) upstream of the human β-globin gene (4.5 kb BglII–EcoRV) was used to generate transgenic mice by standard procedures.
The µZ: plasmid was constructed by linking the bacterial β-galactosidase gene driven by the mouse hsp68 minimal promoter (Kothary et al., 1989) to the µLCR plasmid (Needham et al., 1992; Tewari et al., 1996). The µZ/HS5 and µZ/SCS plasmids were constructed by flanking µZ with the 3 kb BamHI–EcoRI HS5 fragment whereas in µZ/SCS the SCS and SCS′ fragments (Kellum and Schedl, 1992) were positioned 5′ and 3′ with respect to µZ (Figure 4).
PAC1, -2 and -3 (Figure 5) were generated using homologous recombination in Escherichia coli according to Imam et al. (2000). Modified PACs were used to generated transgenic mice. A detailed description of is given in Supplementary data. Expression of µZ constructs were according to Tewari et al. (1996). DNA FISH was as described (Milot et al., 1996) using cos-LCRε as the probe labeled with digoxigenin.
RNA isolation and S1 nuclease protection assay
Total RNA was isolated with the Trizol reagent (Gibco-BRL) from transgenic and wild-type mouse adult blood and E10.5 yolk sac. The S1 procedures and probes (mouse α- and β-major; human γ- and human β-globin) were the same as previously described (Strouboulis et al., 1992; Dillon et al., 1997). Quantitation of the protected fragments was performed by phosphoimager analysis.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We would like to thank Paul Schedl for providing the Drosophila scs and scs′ fragments.This work was supported by the Dutch organization for scientific research NWO (grants to F.G., S.P. and D.M.) and the Medical Research Council, UK.
References
- Antoniou M. (1991) Induction of erythroid-specific expression in murine erythroleukemia (MEL) cell lines. Methods Mol. Biol., 7, 421–434. [DOI] [PubMed] [Google Scholar]
- Bender M.A., Bulger,M., Close,J. and Groudine,M. (2000) β-globin gene switching and DNase I sensitivity of the endogenous β-globin locus in mice do not require the locus control region. Mol. Cell, 5, 387–393. [DOI] [PubMed] [Google Scholar]
- Bungert J., Tanimoto,K., Patel,S., Liu,Q., Fear,M. and Engel,J.D. (1999) Hypersensitive site 2 specifies a unique function within the human β-globin locus control region to stimulate globin gene transcription. Mol. Cell. Biol., 19, 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess-Beusse B., Farrell,C., Gaszner,M., Litt,M., Mutskov,V., Recillas-Targa,F., Simpson,M., West,A. and Felsenfeld,G. (2002) The insulation of genes from external enhancers and silencing chromatin. Proc. Natl Acad. Sci. USA, 99, 16433–16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D., Chakalova,L., Osborne,C.S., Dai,Y.F. and Fraser,P. (2002) Long-range chromatin regulatory interactions in vivo. Nat. Genet., 32, 623–626. [DOI] [PubMed] [Google Scholar]
- Chung J.H., Whiteley,M. and Felsenfeld,G. (1993) A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell, 74, 505–514. [DOI] [PubMed] [Google Scholar]
- Chung J.H., Bell,A.C. and Felsenfeld,G. (1997) Characterization of the chicken β-globin insulator. Proc. Natl Acad. Sci. USA, 94, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon N. and Sabbattini,P. (2000) Functional gene expression domains: defining the functional unit of eukaryotic gene regulation. BioEssays, 22, 657–665. [DOI] [PubMed] [Google Scholar]
- Dillon N., Trimborn,T., Strouboulis,J., Fraser,P. and Grosveld,F. (1997) The effect of distance on long-range chromatin interactions. Mol. Cell, 1, 131–139. [DOI] [PubMed] [Google Scholar]
- Emery D.W., Yannaki,E., Tubb,J. and Stamatoyannopoulos,G. (2000) A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc. Natl Acad. Sci. USA, 97, 9150–9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery D.W., Yannaki,E., Tubb,J., Nishino,T., Li,Q. and Stamatoyannopoulos,G. (2002) Development of virus vectors for gene therapy of β chain hemoglobinopathies: flanking with a chromatin insulator reduces γ-globin gene silencing in vivo. Blood, 100, 2012–2019. [DOI] [PubMed] [Google Scholar]
- Epner E. et al. (1998) The β-globin LCR is not necessary for an open chromatin structure or developmentally regulated transcription of the native mouse β-globin locus. Mol. Cell, 2, 447–455. [DOI] [PubMed] [Google Scholar]
- Farrell C.M., West,A.G. and Felsenfeld,G. (2002) Conserved CTCF insulator elements flank the mouse and human β-globin loci. Mol. Cell. Biol., 22, 3820–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W.C., Takegawa,S., Papayannopoulou,T., Stamatoyannopoulos,G. and Groudine,M. (1987) Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res., 15, 10159–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W.C., Epner,E., Driscoll,M.C., Enver,T., Brice,M., Papayannopoulou,T. and Groudine,M. (1990) A deletion of the human β-globin locus activation region causes a major alteration in chromatin structure and replication across the entire β-globin locus. Genes Dev., 4, 1637–1649. [DOI] [PubMed] [Google Scholar]
- Fraser P., Pruzina,S., Antoniou,M. and Grosveld,F. (1993) Each hypersensitive site of the human β-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev., 7, 106–113. [DOI] [PubMed] [Google Scholar]
- Gillemans N., Tewari,R., Lindeboom,F., Rottier,R., de Wit,T., Wijgerde,M., Grosveld,F. and Philipsen,S. (1998) Altered DNA-binding specificity mutants of EKLF and Sp1 show that EKLF is an activator of the β-globin locus control region in vivo. Genes Dev., 12, 2863–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D.R., Wilson,F.D., Lang,G. and Kioussis,D. (1989) Human CD2 3′-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell, 56, 979–986. [DOI] [PubMed] [Google Scholar]
- Gribnau J., Diderich,K., Pruzina,S., Calzolari,R. and Fraser,P. (2000) Intergenic transcription and developmental remodeling of chromatin subdomains in the human β-globin locus. Mol. Cell, 5, 377–386. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft,G.B., Greaves,D.R. and Kollias,G. (1987) Position-independent, high-level expression of the human β-globin gene in transgenic mice. Cell, 51, 975–985. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Whyatt,D., Fraser,P., Yannoutsos,N., Greaves,D., Dillon,N. and Grosveld,F. (1991) Importance of globin gene order for correct developmental expression. Genes Dev., 5, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Hardison R., Slightom,J.L., Gumucio,D.L., Goodman,M., Stojanovic,N. and Miller,W. (1997) Locus control regions of mammalian β-globin gene clusters: combining phylogenetic analyses and experimental results to gain functional insights. Gene, 205, 73–94. [DOI] [PubMed] [Google Scholar]
- Hebbes T.R., Clayton,A.L., Thorne,A.W. and Crane-Robinson,C. (1994) Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken β-globin chromosomal domain. EMBO J., 13, 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam A.M., Patrinos,G.P., de Krom,M., Bottardi,S., Janssens,R.J., Katsantoni,E., Wai,A.W., Sherratt,D.J. and Grosveld,F.G. (2000) Modification of human β-globin locus PAC clones by homologous recombination in Escherichia coli. Nucleic Acids Res., 28, E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R. and Schedl,P. (1991) A position-effect assay for boundaries of higher order chromosomal domains. Cell, 64, 941–950. [DOI] [PubMed] [Google Scholar]
- Kellum R. and Schedl,P. (1992) A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol. Cell. Biol., 12, 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Vanin,E., deLange,T., Flavell,R.A. and Grosveld,F.G. (1983) β-globin gene inactivation by DNA translocation in γβ-thalassaemia. Nature, 306, 662–666. [DOI] [PubMed] [Google Scholar]
- Kmita M., Fraudeau,N., Herault,Y. and Duboule,D. (2002) Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature, 420, 145–150. [DOI] [PubMed] [Google Scholar]
- Kong R.Y., Kwan,K.M., Lau,E.T., Thomas,J.T., Boot-Handford,R.P., Grant,M.E. and Cheah,K.S. (1993) Intron–exon structure, alternative use of promoter and expression of the mouse collagen X gene, Col10a-1. Eur. J. Biochem., 213, 99–111. [DOI] [PubMed] [Google Scholar]
- Kothary R., Clapoff,S., Darling,S., Perry,M.D., Moran,L.A. and Rossant,J. (1989) Inducible expression of an hsp68–lacZ hybrid gene in transgenic mice. Development, 105, 707–714. [DOI] [PubMed] [Google Scholar]
- Levings P.P. and Bungert,J. (2002) The human β-globin locus control region. Eur. J. Biochem., 269, 1589–1599. [DOI] [PubMed] [Google Scholar]
- Li Q. and Stamatoyannopoulos,G. (1994) Hypersensitive site 5 of the human beta locus control region functions as a chromatin insulator. Blood, 84, 1399–1401. [PubMed] [Google Scholar]
- Li Q., Zhang,M., Duan,Z. and Stamatoyannopoulos,G. (1999) Structural analysis and mapping of DNase I hypersensitivity of HS5 of the β-globin locus control region. Genomics, 61, 183–193. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang,M., Han,H., Rohde,A. and Stamatoyannopoulos,G. (2002) Evidence that DNase I hypersensitive site 5 of the human β-globin locus control region functions as a chromosomal insulator in transgenic mice. Nucleic Acids Res., 30, 2484–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum M.H. and Grosveld,F. (1990) An in vitro globin gene switching model based on differentiated embryonic stem cells. Genes Dev., 4, 2075–2085. [DOI] [PubMed] [Google Scholar]
- Litt M.D., Simpson,M., Gaszner,M., Allis,C.D. and Felsenfeld,G. (2001a) Correlation between histone lysine methylation and developmental changes at the chicken β-globin locus. Science, 293, 2453–2455. [DOI] [PubMed] [Google Scholar]
- Litt M.D., Simpson,M., Recillas-Targa,F., Prioleau,M.N. and Felsenfeld,G. (2001b) Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J., 20, 2224–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q., Bengra,C., Li,C., Kutlar,F. and Tuan,D. (1998) A long terminal repeat of the human endogenous retrovirus ERV-9 is located in the 5′ boundary area of the human β-globin locus control region. Genomics, 54, 542–555. [DOI] [PubMed] [Google Scholar]
- Milot E. et al. (1996) Heterochromatin effects on the frequency and duration of LCR-mediated gene transcription. Cell, 87, 105–114. [DOI] [PubMed] [Google Scholar]
- Needham M., Gooding,C., Hudson,K., Antoniou,M., Grosveld,F. and Hollis,M. (1992) LCR/MEL: a versatile system for high-level expression of heterologous proteins in erythroid cells. Nucleic Acids Res., 20, 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy S., Elefanty,A.G., Visvader,J., Bath,M.L., Harris,A.W. and Adams,J.M. (1998) Transcriptional regulation of vav, a gene expressed throughout the hematopoietic compartment. Blood, 91, 419–430. [PubMed] [Google Scholar]
- Peterson K.R. and Stamatoyannopoulos,G. (1993) Role of gene order in developmental control of human γ- and β-globin gene expression. Mol. Cell. Biol., 13, 4836–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipsen S., Talbot,D., Fraser,P. and Grosveld,F. (1990) The β-globin dominant control region: hypersensitive site 2. EMBO J., 9, 2159–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipsen S., Pruzina,S. and Grosveld,F. (1993) The minimal requirements for activity in transgenic mice of hypersensitive site 3 of the β globin locus control region. EMBO J., 12, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaart M.J., Recillas-Targa,F. and Felsenfeld,G. (1998) Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev., 12, 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioleau M.N., Nony,P., Simpson,M. and Felsenfeld,G. (1999) An insulator element and condensed chromatin region separate the chicken β-globin locus from an independently regulated erythroid-specific folate receptor gene. EMBO J., 18, 4035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recillas-Targa F., Pikaart,M.J., Burgess-Beusse,B., Bell,A.C., Litt,M.D., West,A.G., Gaszner,M. and Felsenfeld,G. (2002) Position-effect protection and enhancer blocking by the chicken β-globin insulator are separable activities. Proc. Natl Acad. Sci. USA, 99, 6883–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik A., Telling,A., Zitnik,G., Cimbora,D., Epner,E. and Groudine,M. (1998) The locus control region is necessary for gene expression in the human β-globin locus but not the maintenance of an open chromatin structure in erythroid cells. Mol. Cell. Biol., 18, 5992–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose L., Lounnas,V., Ehrlich,L., Buchholz,F., Wade,R. and Stewart,A.F. (1998) Comparative kinetic analysis of FLP and cre recombinases: mathematical models for DNA binding and recombination. J. Mol. Biol., 284, 363–384. [DOI] [PubMed] [Google Scholar]
- Roseman R.R., Pirrotta,V. and Geyer,P.K. (1993) The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J., 12, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K. and Miyazaki,J. (1997) A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem. Biophys. Res. Commun., 237, 318–324. [DOI] [PubMed] [Google Scholar]
- Southwood C.M., Downs,K.M. and Bieker,J.J. (1996) Erythroid Kruppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev. Dyn., 206, 248–259. [DOI] [PubMed] [Google Scholar]
- Strouboulis J., Dillon,N. and Grosveld,F. (1992) Developmental regulation of a complete 70-kb human β-globin locus in transgenic mice. Genes Dev., 6, 1857–1864. [DOI] [PubMed] [Google Scholar]
- Talbot D., Philipsen,S., Fraser,P. and Grosveld,F. (1990) Detailed analysis of the site 3 region of the human β-globin dominant control region. EMBO J., 9, 2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto K., Liu,Q., Bungert,J. and Engel,J.D. (1999a) Effects of altered gene order or orientation of the locus control region on human β-globin gene expression in mice. Nature, 398, 344–348. [DOI] [PubMed] [Google Scholar]
- Tanimoto K., Liu,Q., Bungert,J. and Engel,J.D. (1999b) The polyoma virus enhancer cannot substitute for DNase I core hypersensitive sites 2–4 in the human β-globin LCR. Nucleic Acids Res., 27, 3130–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R., Gillemans,N., Harper,A., Wijgerde,M., Zafarana,G., Drabek,D., Grosveld,F. and Philipsen,S. (1996) The human β-globin locus control region confers an early embryonic erythroid-specific expression pattern to a basic promoter driving the bacterial lacZ gene. Development, 122, 3991–3999. [DOI] [PubMed] [Google Scholar]
- Tewari R., Gillemans,N., Wijgerde,M., Nuez,B., von Lindern,M., Grosveld,F. and Philipsen,S. (1998) Erythroid Kruppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5′HS3 of the β-globin locus control region. EMBO J., 17, 2334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B., Palstra,R.J., Splinter,E., Grosveld,F. and de Laat,W. (2002) Looping and Interaction between Hypersensitive Sites in the Active beta- globin Locus. Mol. Cell, 10, 1453–65. [DOI] [PubMed] [Google Scholar]
- Tuan D. and London,I.M. (1984) Mapping of DNase I-hypersensitive sites in the upstream DNA of human embryonic ε-globin gene in K562 leukemia cells. Proc. Natl Acad. Sci. USA, 81, 2718–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon,W., Li,Q. and London,I.M. (1985) The ‘beta-like-globin’ gene domain in human erythroid cells. Proc. Natl Acad. Sci. USA, 82, 6384–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijgerde M., Gribnau,J., Trimborn,T., Nuez,B., Philipsen,S., Grosveld,F. and Fraser,P. (1996) The role of EKLF in human β-globin gene competition. Genes Dev., 10, 2894–2902. [DOI] [PubMed] [Google Scholar]
- Wijgerde M., Grosveld,F. and Fraser,P. (1995) Transcription complex stability and chromatin dynamics in vivo. Nature, 377, 209–213. [DOI] [PubMed] [Google Scholar]
- Wolffe A.P. (1996) Chromatin and gene regulation at the onset of embryonic development. Reprod. Nutr. Dev., 36, 581–606. [PubMed] [Google Scholar]
- Zhou J., Barolo,S., Szymanski,P. and Levine,M. (1996) The Fab-7 element of the bithorax complex attenuates enhancer-promoter interactions in the Drosophila embryo. Genes Dev., 10, 3195–3201. [DOI] [PubMed] [Google Scholar]