Abstract
MEKK1-deficient mice show an eye open at birth phenotype caused by impairment in embryonic eyelid closure. MEK kinase 1 (MEKK1) is highly expressed in the growing tip of the eyelid epithelium, which displays loose cell–cell contacts and prominent F-actin fibers in wild-type mice, but compact cell contacts, lack of polymerized actin and a concomitant impairment in c-Jun N-terminal phosphorylation in MEKK1-deficient mice. In cultured keratinocytes, MEKK1 is essential for JNK activation by TGF-β and activin, but not by TGF-α. MEKK1-driven JNK activation is required for actin stress fiber formation, c-Jun phosphorylation and cell migration. However, MEKK1 ablation does not impair other TGF-β/activin functions, such as nuclear translocation of Smad4. These results establish a specific role for the MEKK1–JNK cascade in transmission of TGF-β and activin signals that control epithelial cell movement, providing the mechanistic basis for the regulation of eyelid closure by MEKK1. This study also suggests that the signaling mechanisms that control eyelid closure in mammals and dorsal closure in Drosophila are evolutionarily conserved.
Keywords: actin stress fiber/embryonic eyelid closure/epithelial cell migration/MEKK1–JNK pathway/TGF-β/activin signaling
Introduction
There are three major groups of mitogen-activated protein kinases (MAPKs) that are conserved between insects and mammals, including the extracellular signal-regulated kinases (ERKs), the c-Jun N-terminal kinases (JNKs) and p38 (Davis, 2000). MEKK1 is a member of the MAPK kinase kinase (MAPKKK) family that activates MAPKs through the MAPKKK–MAPKK signaling cascade (Karin, 1996). As an upstream regulator of several MAPK pathways, MEKK1 has been implicated in diverse and cell-type-specific biological responses, including apoptosis induced by stress stimuli (Gibson et al., 2002), T-cell activation (Tao et al., 2002), cardiac hypertrophy (Minamino et al., 2002) and keratinocyte terminal differentiation (Vuong et al., 2000).
When overexpressed, MEKK1 leads to activation of all three MAPK pathways, albeit with a strong preference for stimulation of the JNK pathway. MEKK1 directly interacts with and phosphorylates the JNK activating kinases, JNKK1/MKK4 and JNKK2/MKK7 (Yan et al., 1994; Lin et al., 1995; Wu et al., 1997; Tournier et al., 1997; Xia et al., 1998). The endogenous MEKK1 is an important JNK activator, as its ablation selectively blocks JNK activation by a subset of physiological and stress stimuli, but has only a marginal or zero effect on the activation of ERK and p38 (Yujiri et al., 1998; Xia et al., 2000). Hence, MEKK1 may regulate certain cellular processes that depend on JNK activation in vivo.
The in vivo role of the JNK pathway has been studied in the fruit fly, in which Drosophila JNK (DJNK) controls embryonic dorsal closure, involving lateral epithelial sheet movements, to close an opening in the dorsal epidermis (Glise and Noselli, 1997; Sluss and Davis, 1997). A JNK-regulated signaling network controls AP-1 transcription factors and through them the expression of the growth factor Decapentaplegic (DPP), a Drosophila homolog of TGF-β, which in turn induces epithelial cell cytoskeletal reorganization and shape changes necessary for dorsal closure (Jacinto et al., 2002). In the mouse, dual ablation of JNK1 and JNK2 results in lethality at embryonic day 12 (E12) due to defective neural tube closure (Kuan et al., 1999). Although there is superficial resemblance between the open neural tube phenotype and the dorsal closure defects exhibited by DJNK mutants, the two processes are mechanistically distinct, as neural tube closure is determined by JNK-mediated apoptosis of lateral neural folds. Many of the proteins regulating dorsal closure have been implicated in epithelial cell movements in other organisms, but until now a mammalian process that is mechanistically similar to dorsal closure in Drosophila, exhibiting dependence on JNK-regulated actin polymerization and c-Jun function, has not been identified.
Recent studies on mouse cells deficient in MEKK1 activity have uncovered a unique function of this protein kinase in cell migration. Two knockout strategies were used to generate MEKK1-deficient cells. In one system, the translation initiation site for MEKK1 was deleted, resulting in a null Mekk1– allele and absence of the entire MEKK1 polypeptide (Yujiri et al., 1998). MEKK1-null mouse embryonic fibroblasts (MEFs) display reduced cell motility, although this MEKK1-dependent function is independent of JNK activity. In another system, the Mekk1 locus was disrupted by replacing the exons coding for the MEKK1 kinase domain with the bacterial LacZ gene, generating a Mekk1ΔKD allele which specifies expression of an MEKK1–β-galactosidase fusion protein containing the first 1188 amino acids of MEKK1 but lacking the kinase domain (Xia et al., 2000). Embryonic stem (ES) cells lacking MEKK1 kinase domain exhibit defective serum-induced cell migration and are also impaired in JNK activation. Together, these results suggest that MEKK1 is likely to affect the machinery that controls cell motility and that this function depends on its kinase domain.
MEKK1-deficient mice complete embryonic development and are born with relatively normal appearance, except for the eye open at birth (EOB) phenotype (Yujiri et al., 2000). Mouse eyelid formation initiates at E13, with folds of surface ectoderm adjacent to the developing eye. Eyelid closure occurs between E15.5 and E16.5, when the outermost layer of the eyelid epidermis starts to move toward the center of the eye and covers the entire ocular surface, fusing to form a closed eyelid as a protective barrier over the cornea (Harris and Juriloff, 1986; Findlater et al., 1993). The morphological features of the epithelial sheet movements associated with mouse eyelid closure resemble the events that occur during dorsal closure in the fruit fly.
To understand the signaling mechanisms of MEKK1 during eyelid closure, we compared the mechanics of this process in wt and Mekk1ΔKD/ΔKD mice (Xia et al., 2000). We find that MEKK1 is expressed at high levels at the migrating edge of the eyelid epithelium, where it is required for F-actin formation. MEKK1 is also required for TGF-β/activin-induced actin stress fiber formation and migration of cultured keratinocytes, therefore uncovering an important mechanism by which MEKK1 regulates epithelium sheet movement and eyelid closure. Studies utilizing a specific JNK inhibitor strongly suggest that this activity of MEKK1 depends on JNK activation which also leads to c-Jun N-terminal phosphorylation. These results also suggest that mammalian eyelid closure is mechanistically similar to dorsal closure in Drosophila and outline an evolutionary conservation in the developmental function of the JNK signaling cascade in two distinct biological systems.
Results
MEKK1 is required for embryonic eyelid closure
To generate homozygous MEKK1-deficient mice, we intercrossed mice heterozygous for the Mekk1ΔKD allele (Xia et al., 2000). Genotypic analysis identified 132 F2 mice homozygous for the Mekk1ΔKD allele, all of which exhibited EOB. In contrast, a total of 445 wt and Mekk1+/ΔKD heterozygote mice were born with closed eyelids (Figure 1A).
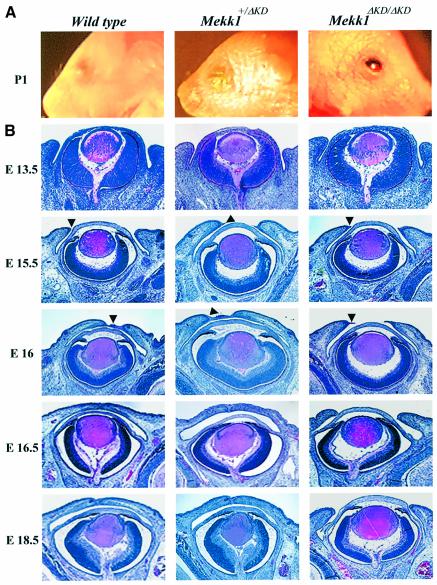
Fig. 1. MEKK1 ablation causes impairment in embryonic eyelid closure. (A) Photograph of the eyes of wt (left panel), Mekk1+/ΔKD (middle panel) and Mekk1ΔKD/ΔKD mice (right panel) at postnatal day 1, showing the EOB phenotype in Mekk1ΔKD/ΔKD mice. (B) H&E staining the coronal eye sections from wt (left panels), Mekk1+/ΔKD (middle panels) and Mekk1ΔKD/ΔKD (right panels) fetuses of various gestational ages (E13.5–E18.5). Arrowheads point to the developing eyelid tip and impaired eyelid closure is observed in Mekk1ΔKD/ΔKD fetuses at E16.
The EOB phenotype had been previously observed in Mekk1–/– mice (Yujiri et al., 2000), although no histological or molecular analyses of these mice were advanced to develop a mechanistic explanation of the phenotype. To determine why loss of MEKK1 activity results in this defect, we first performed histological analyses on the developing eye of the Mekk1ΔKD/ΔKD mice. Prior to E15.5, the eyelid morphology of Mekk1ΔKD/ΔKD fetuses appeared to be identical to that of their wt littermates, displaying emergence of the eyelids at E13.5, as well as extension at E15.5. By E16, the ocular surface of the wt and Mekk1+/ΔKD heterozygotes was covered by a thin epithelium, extending from the tip of the developing eyelid (Figure 1B). In contrast, the eyelid of the homozygous mutants failed to move forward, leaving the ocular surface exposed. By E16.5, wt and heterozygous fetuses, but not Mekk1ΔKD/ΔKD fetuses, formed completely closed eyelids. Other eye structures, including lens, retina and cornea, were morphologically normal in the homozygous mutant fetuses until birth.
MEKK1 is expressed in ocular surface tissues during development
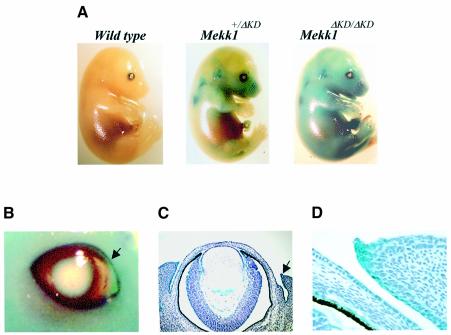
To identify the specific role for MEKK1 in regulation of eyelid closure, we studied MEKK1 expression patterns during mouse fetal development. The Mekk1ΔKD allele generates a fusion protein, containing the N-terminal non-catalytic domain of MEKK1 fused to β-galactosidase, whose expression is controlled by the normal Mekk1 promoter (Xia et al., 2000). This fusion protein is expressed as a polypeptide of approximately 250 kDa, which was previously identified in Mekk1+/ΔKD and Mekk1ΔKD/ΔKD cells by western blotting. In vivo expression of the MEKK1–β-gal fusion protein can be detected by whole-mount staining of mouse embryos with X-gal, a β-galactosidase substrate. The results showed β-galactosidase activities in fetuses of various gestational ages that are Mekk1ΔKD heterozygotes and homozygotes, but not in wt littermates, confirming that the β-galactosidase activity was derived from the Mekk1ΔKD allele (Figure 2A). X-gal-positive cells distributed over almost the entire embryonic body of E15.5 fetuses and accumulated at eyelids, whisker follicles, ear and maxillary bone (Figure 2A and B). The in vivo expression of the fusion protein in Mekk1ΔKD/ΔKD homozygotes, albeit at an elevated level, followed a pattern similar to that in Mekk1+/ΔKD heterozygotes, suggesting that lack of MEKK1 activity in the mutants did not affect expression of the Mekk1ΔKD allele.
Fig. 2. MEKK1 expression during embryonic development. (A) wt, Mekk1+/ΔKD and Mekk1ΔKD/ΔKD fetuses of E15.5 were stained for β-galactosidase expression. Photographs were taken at 7.5× magnification. (B) β-galactosidase expression in the eye of E15.5 Mekk1ΔKD/ΔKD fetuses. The arrow indicates the leading edge of the developing eyelid, photographed at 15× magnification. Coronal sections of X-gal-stained E15.5 fetuses, counterstained with hematoxylin and photographed at (C) 100× and (D) 200× magnification. β-galactosidase expressing cells (blue) were observed in a variety of eye tissues, including eyelid, conjunctiva, iris and ciliary body, retina and lens epithelium.
Histological analyses of X-gal-stained embryos showed the expression of the fusion protein in numerous ocular tissues, predominantly in the epithelium of the eyelid tip, conjunctiva and skin (Figure 2C and D). MEKK1-positive cells were also detected in the ciliary body, iris and lens, as well as in retinal ganglion cell layers.
Morphology of eyelid epithelium in Mekk1ΔKD/ΔKD fetuses
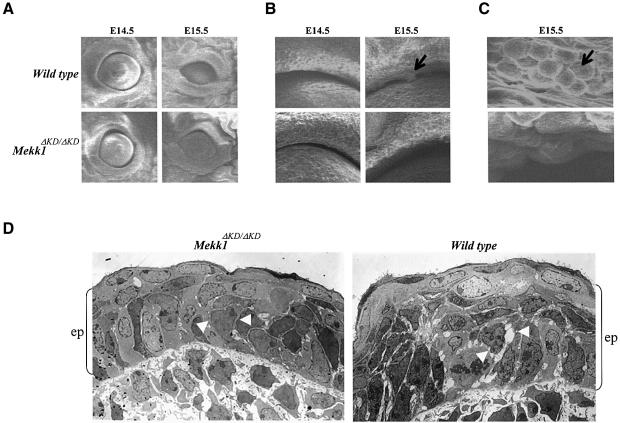
To search for morphological changes in the eyelid epithelium, we performed scanning electron microscopy on eyes of E14.5 and E15.5 wt and Mekk1ΔKD/ΔKD fetuses. At E14.5, both wt and mutant fetuses displayed eye opening in similar oval shapes and sizes with no distinct morphological differences at the eyelid margin (Figure 3A and B). Differences between wt and Mekk1ΔKD/ΔKD fetuses became evident at E15.5, when the eye opening was reduced in wt, but much less so in the mutant. The clumps of round cells along the eyelid margin in wt were undetectable in Mekk1ΔKD/ΔKD fetuses (Figure 3B and C).
Fig. 3. MEKK1 ablation affects the eyelid epithelium morphology. Eye samples of wt and Mekk1ΔKD/ΔKD fetuses at the indicated ages were studied by scanning electron microscopy. Photographs taken at (A) low (60×), (B) medium (400×) and (C) high (1200×) magnification showing clumps of round cells along the eyelid margin (arrows) present in wt but not in Mekk1ΔKD/ΔKD E15.5 fetuses. (D) Transmission electron microscopy of the eyelid tip epithelium from wt and Mekk1ΔKD/ΔKD E15.5 fetuses. The wt eyelid epithelium (ep), delineated by the brackets, is thicker than its Mekk1ΔKD/ΔKD counterpart. The epithelium of the wt eyelid displays reduced cell–cell contacts and increased intercellular spaces (white arrowheads). In contrast, the epithelium of Mekk1ΔKD/ΔKD eyelids displays tight epithelial cell interactions (white arrowheads).
Transmission electron microscopy was also used to examine the morphology of epithelium at the developing eyelid tips. One striking difference was the thickness of the eyelid epithelium, with the wt epithelium being considerably thicker than that of the mutant (Figure 3D). The decreased thickness of the eyelid epithelium in Mekk1ΔKD/ΔKD fetuses was not associated with a reduced number of cell layers; rather, it appeared to be derived from architectural differences between the normal and mutant epithelium. The wt epithelium exhibited loose cell–cell interactions, with the presence of numerous large intercellular spaces, while the epithelium of the mutant displayed tight cell–cell contacts with significantly fewer and smaller intercellular spaces. Hence, MEKK1 is clearly needed for certain morphological changes of eyelid epithelium during development.
MEKK1 is required for epidermal keratinocyte migration induced by TGF-β/activin, but not by TGF-α
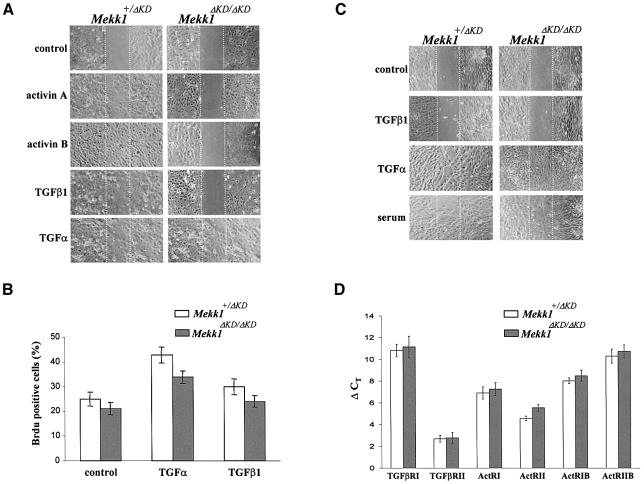
The above data suggest that impaired eyelid closure in Mekk1ΔKD/ΔKD mice may result from defects of eyelid epithelium function. Many factors critical for embryonic eyelid closure, such as transforming growth factor-α (TGF-α) and activin βB (Vassalli et al., 1994; Berkowitz et al., 1996), are also regulators of epithelial cell migration. Activin βB is a member of the transforming growth factor-β (TGF-β) superfamily and, like TGF-α, achieves its effect through activation of intracellular signaling pathways. It is possible that MEKK1 functions as an intracellular mediator for signals that control epithelial cell migration required for eyelid closure. As the Mekk1+/ΔKD mice exhibit normal development and closed eyelids at birth, one functional Mekk1 allele should be sufficient to support cell migration that results in eyelid closure. For this reason, we used Mekk1ΔKD/ΔKD and Mekk1+/ΔKD keratinocytes, the major constituents of eyelid epidermis, for an in vitro wound-healing assay to assess the role of MEKK1 in growth-factor-induced cell migration. Under growth-factor-deprived conditions, neither Mekk1+/ΔKD nor Mekk1ΔKD/ΔKD keratinocytes were effective in wound closure (Figure 4A). We then tested TGF-α and several members of the TGF-β family, including activin A, activin B and TGF-β1, for their effects on keratinocyte migration (Bakin et al., 2000; Kim et al., 2001; Beck et al., 2003). All factors, with TGF-α and activin B being more potent than TGF-β1 and activin A, induced marked migration of Mekk1+/ΔKD keratinocytes, causing wound closure over a 24 h period. Mekk1ΔKD/ΔKD keratinocytes were fully responsive to TGF-α, but did not respond to any of the TGF-β family members. Thus, MEKK1 appears to be critical for keratinocyte migration induced by TGF-β family members, but not that induced by TGF-α. Both TGF-α and TGF-β exhibited slight proliferative effects on keratinocytes that were independent of MEKK1 (Figure 4B).
Fig. 4. MEKK1 is required for keratinocyte migration induced by TGF-β/activin. Confluent monolayers of mouse epidermal keratinocytes (A) or dermal fibroblasts (C) were subjected to in vitro wound-healing assays in medium without growth factors (control) or with activin A (5 ng/ml), activin B (5 ng/ml), TGF–β1 (10 ng/ml), TGF-α (10 ng/ml) or fetal calf serum (5%), as indicated. Photographs were taken immediately and 24 h after wounding; only the 24 h time point is shown. (B) Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes were labeled with BrdU and BrdU-positive cells were identified by indirect immunofluorescence. A total of 200 cells per experimental condition were examined and error bars indicate 95% confidence limits. (D) Total RNA isolated from Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes were examined by real-time RT–PCR for the expression of TGF-β/activin receptors, as indicated. Each experiment was performed in triplicate and the ordinate (ΔCT) represents the number of cycles needed to reach an arbitrary amplification threshold value normalized to GAPDH mRNA in the same sample.
To determine the cell-type specificity of MEKK1 functions, we studied the migration of dermal fibroblasts, another major constituent of the developing eyelids. Dermal fibroblast cultures of both genotypes responded equally well to serum factors and TGF-α, reaching complete wound closure within 24 h (Figure 4C). Neither Mekk1+/ΔKD nor Mekk1ΔKD/ΔKD fibroblasts were responsive to TGF-β1 treatment. Together, these results suggest a specific role for MEKK1 in TGF-β-induced migration of epidermal keratinocytes, but not fibroblasts.
MEKK1 regulates actin polymerization in cultured keratinocytes and developing eyelid epithelium
Actin reorganization is a critical cellular event required for cell migration. If MEKK1 does indeed play a role in keratinocyte migration, we may expect to find defective actin reorganization in Mekk1ΔKD/ΔKD cells. Therefore we examined F-actin in Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes treated with TGF-α and activin B, a representative of the TGF-β family and a potent inducer of keratinocyte migration. TGF-α caused a rapid induction of F-actin in both Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes, with observable actin stress fibers formed in 95% of Mekk1+/ΔKD cells (63 of 66) and 91% of Mekk1ΔKD/ΔKD cells (43 of 47) (Figure 5A). A similar effect of activin B on F-actin was evident in 90% of Mekk1+/ΔKD keratinocytes (51 of 56), but was completely absent in all Mekk1ΔKD/ΔKD keratinocytes that were examined (none of 49). Activin A and TGF–β1 exhibited similar effects to activin B (data not shown). In the absence of MEKK1, keratinocytes were not responsive to TGF-β/activin, with actin remaining condensed at the cell cortex and cell–cell junctions, similar to what was observed in untreated controls. F-actin induction by TGF-α or activin B was a rapid response, occurring within 30 min and persisting for up to 6 h, followed by actin depolymerization 12 h after treatment (data not shown).
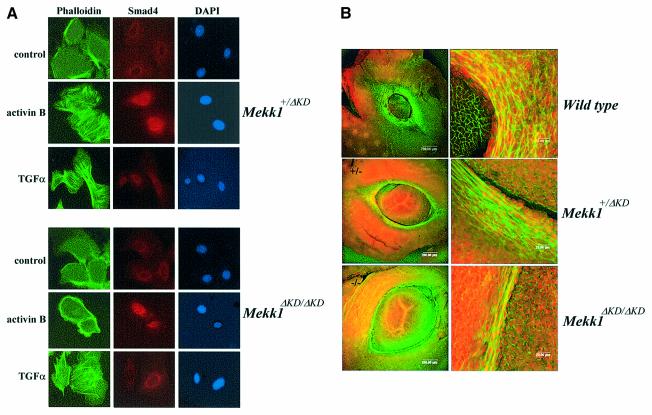
Fig. 5. MEKK1 controls actin stress fiber formation in epidermal keratinocytes and in the developing eyelid epithelium. (A) Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes were maintained in growth-factor-free medium for 24 h before treatment with activin B (5 ng/ml) and TGF-α (10 ng/ml) for 2 h. Immunostaining was performed with FITC–phalloidin (green) for F-actin, anti-Smad4 and Alexa Fluor (red) for Smad4 and DAPI (blue) for nuclei. Activin B-induced actin stress fiber formation takes place in Mekk1+/ΔKD, but not in Mekk1ΔKD/ΔKD keratinocytes whereas, Smad4 nuclear translocation is unaffected by MEKK1 ablation. Photographs were taken at 600× magnification under fluorescence light. (B) Whole-mount staining of the eyes from wt, Mekk1+/ΔKD and Mekk1ΔKD/ΔKD fetuses at E15.5 was performed using FITC–phalloidin and propidium iodide for F–actin and DNA, respectively. The FITC–phalloidin binding to F-actin is clearly decreased in eyelids of Mekk1ΔKD/ΔKD fetuses. Images were captured by laser scanning microscope at low (left panels) and higher (right panels) magnifications.
To test whether MEKK1 also regulates actin polymerization in vivo in the developing eyelid epithelium, we examined formation of actin filaments in eyelid tissues of E15.5 fetuses. In both wt and Mekk1+/ΔKD fetuses, eyelid epithelial cells developed prominent F-actin networks as demonstrated by in situ phalloidin staining (Figure 5B). While large numbers of epithelial cells in wt and Mekk1+/ΔKD fetuses, spreading to wide areas of the developing eyelid, exhibited prominent F-actin fibers, only a few cells in the homozygous mutant, mostly confined to a single cell layer at the eyelid tip, displayed formation of actin cables. These data demonstrate that MEKK1 regulates actin stress fiber formation in epithelial cells of the developing eyelid, likely associated with epithelium movement and eyelid closure.
Lack of a response of Mekk1ΔKD/ΔKD keratinocytes to TGF-β/activin may be caused by defective expression of TGF-β/activin receptors. To examine this possibility, we used real-time RT-PCR to measure the mRNA levels of TGF-β receptors in RNA isolated from Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes. In this particular cell type, some receptors, such as ACTRII and TGF-βRII, showed a higher level of expression than others after normalization to GAPDH mRNA in the same sample; however, none of their expression was affected by MEKK1 ablation (Figure 4D). Ligand-induced receptor activation resulting in Smad4 nuclear translocation was evident in both Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes exposed to activin B, a pattern distinct from either the untreated cells or cells exposed to TGF-α in which Smad4 was mainly localized in the perinuclear region of the cells (Figure 5A). These results suggest that MEKK1 ablation does not affect activin B receptor expression or its signaling to the Smad transcription factors and that MEKK1 is specifically required for transducing TGF-β/activin signals that control F-actin formation and epithelial cell migration.
JNK activity is essential for TGF-β/activin-induced actin polymerization and embryonic eyelid closure
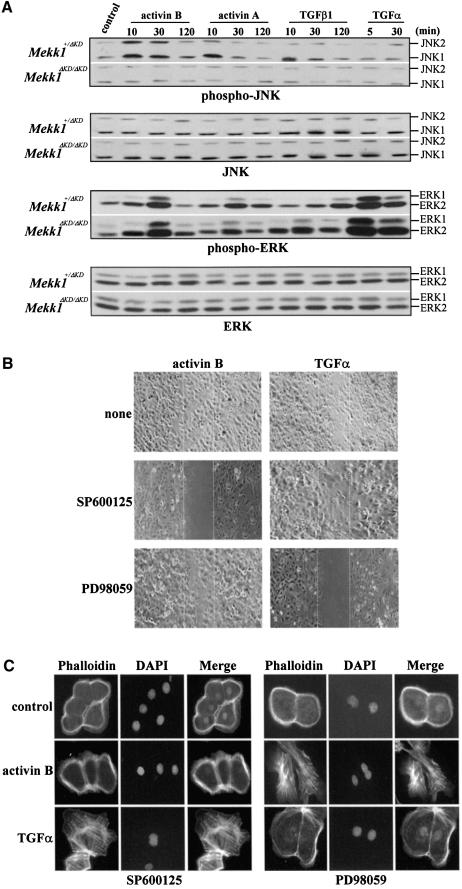
To elucidate the downstream events of MEKK1 in TGF-β/activin signaling, we examined activation of JNK, ERK and p38 by members of the TGF-β family and TGF-α. In Mekk1+/ΔKD keratinocytes, activin B caused an immediate and transient JNK activation, which was detectable at 15 min and reduced to the basal level at 2 h of treatment. Activin A and TGF-β1 also activate JNK in a similar manner but to a lesser extent than activin B (Figure 6A). More importantly, JNK activation by any of the TGF-β/activin peptides was completely abolished in Mekk1ΔKD/ΔKD keratinocytes, wherein JNK expression was unaltered. ERK phosphorylation was not significantly affected by MEKK1 ablation, agreeing with previous findings in ES cells (Xia et al., 2000). Although TGF-α activated ERK in both Mekk1+/ΔKD and Mekk1ΔKD/ΔKD cells, it was unable to activate JNK (Figure 6A). p38 activity was undetectable in cells exposed to any of the growth factors. We conclude from these experiments that MEKK1 is essential for transducing signals from TGF-β/activin receptors, but not from TGF-α, to JNK; it has little, if any, role in the activation of ERK and p38 in the keratinocyte system by these factors.
Fig. 6. The TGF-β/activin-induced MEKK1–JNK pathway is required for actin polymerization and epithelial cell migration. (A) JNK activation by TGF-β/activin is mediated through MEKK1. The Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes were treated by various growth factors for the indicated times and cell lysates were assessed for phosphorylation and expression of JNK and ERK by western blotting using specific antibodies. JNK phosphorylation induced by TGF-β/activin was completely abolished by MEKK1 ablation, while ERK phosphorylation induced by TGF-α and TGF-β/activin was only marginally affected. Epithelial cell migration (B) and actin stress fiber formation (C) induced by activin B require JNK, while the response to TGF-α requires ERK. Mekk1+/ΔKD keratinocytes were deprived of growth factors for 24 h and pretreated with the JNK inhibitor SP600125 (5 µM) and the MEK inhibitor PD98059 (5 µM) for 0.5 h. The cells were cultured in medium without growth factors (control) or with either TGF-α (10 ng/ml) or activin B (5 ng/ml) for (B) 24 h for the in vitro wound healing assay and (C) 2 h for detection of F-actin formation by fluorescence staining. Wound closure and actin stress fiber formation induced by activin B were blocked by the JNK inhibitor, while the response to TGF-α was prevented by the ERK inhibitor.
If JNK is important for TGF-β signaling, its inhibition should prevent TGF-β-induced cell functions, such as epithelial cell migration and actin stress fiber formation. Indeed, pretreatment of the Mekk1+/ΔKD keratinocytes with SP600125, an inhibitor of JNK activity (Han et al., 2001), almost completely abolished activin-B-induced, but not TGF-α-induced, keratinocyte migration and in vitro wound closure (Figure 6B). The same inhibitor also markedly suppressed actin polymerization, with only two of 66 cells (3%) remaining positive for actin stress fibers (Figure 6C). On the other hand, inhibition of ERK activation with PD98059, a MEK inhibitor (Kultz et al., 1998), did not produce such an effect. In contrast, the ERK inhibitor prevented TGF-α-induced wound closure of keratinocytes and abolished the cell response to TGF-α, with 95% of the cells(85 of 90) scoring negative for actin stress fibers. These data suggest a specificity of MAPKs in mediating cell signals that control actin polymerization and keratinocyte migration: the TGF-β/activin signal is mediated through the MEKK1–JNK pathway, while the TGF-α signal is mediated through the ERK pathway.
MEKK1 regulates c-Jun N-terminal phosphorylation in developing eyelid epithelium
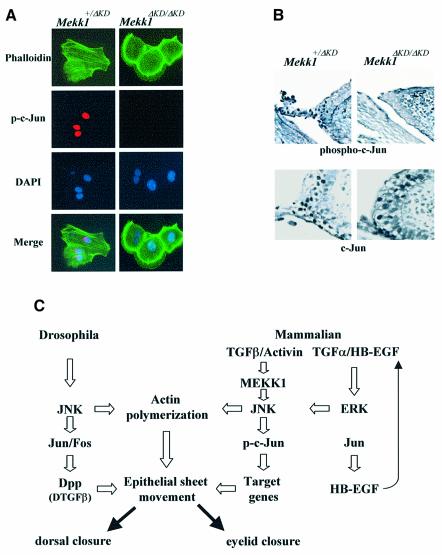
One of the well-defined nuclear effects of JNK is the phosphorylation of the c-Jun transcription factor and potentiation of its transcriptional activity (Hibi et al., 1993). As an eyelid closure defect was also found in mice with a keratinoctye-specific knockout of c-Jun (Li et al., 2003; Zenz et al., 2003), we inferred that the MEKK1–JNK cascade may function through c-Jun, whose phosphorylation could be one of the endpoints of TGF-β/activin signaling. Induction of c-Jun phosphorylation was clearly detectable in Mekk1+/ΔKD, but not Mekk1ΔKD/ΔKD, keratinocytes exposed to activin B. Phosphorylation occurred as early as 2 h and reached 100% by 6 h of activin B exposure but was undetectable in cells treated with TGF-α (Figure 7A and data not shown).
Fig. 7. Involvement of c-Jun N-terminal phosphorylation in activin signaling and eyelid development. (A) MEKK1 is required for activin-B-induced c-Jun N-terminal phosphorylation in keratinocyte. Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes were maintained in growth-factor-free medium for 24 h, followed by treatment with activin B (5 ng/ml) for 2 h. Immunostaining was carried out using anti-phospho-c-Jun (S63), FITC–phalloidin and DAPI for c-Jun phosphorylation, F-actin and nuclei, respectively. Activin-B-induced c-Jun phosphorylation and F-actin are both abolished by MEKK1 ablation. (B) Eye sections of E15.5 Mekk1+/ΔKD and Mekk1ΔKD/ΔKD fetuses were immunostained with anti-phospho-c-Jun (S63) (top panels) or anti-c-Jun (bottom panels). Many cells in the eyelid epithelium of Mekk1+/ΔKD fetuses show positive nuclear phospho-c-Jun staining, while only a few phospho-c-Jun-positive cells are detected in Mekk1ΔKD/ΔKD eyelids. c-Jun-positive cells are detected at the similar frequencies in the eyelid epithelia of Mekk1+/ΔKD and Mekk1ΔKD/ΔKD. (C) An evolutionarily conserved JNK pathway regulates mammalian eyelid closure and Drosophila dorsal closure. In mammalian eyelid closure, one pathway involves the MEKK1–JNK cascade, required for receiving and transmitting the TGF-β/activin signal in the control of actin polymerization and c-Jun phosphorylation which is important for epithelial sheet movement. Another pathway is c-Jun controlled expression of HB-EGF, which in turn may activate the EGFR–ERK pathway that also leads to actin polymerization and epithelial sheet movement. In Drosophila, a parallel regulatory mechanism, involving JNK, c-Jun and TGF-β, controls dorsal epithelium actin polymerization and closure. The MEKK1–JNK and the EGFR–ERK pathways are likely mammalian equivalents of the Drosophila JNK-AP-1-TGF-β pathway, representing an evolutionarily conserved signaling mechanism that controls epithelial sheet movement and tissue closure across species.
In the developing eyelid epithelium, c-Jun N-terminal phosphorylation could also be readily detected in Mekk1+/ΔKD fetuses but was substantially reduced in Mekk1ΔKD/ΔKD fetuses (Figure 7B). Expression of the c-Jun protein in the developing eyelid tip epithelium of E15.5 fetuses was not substantially affected by the lack of MEKK1 activity, with similar levels in Mekk1+/ΔKD and Mekk1ΔKD/ΔKD mice. Taken together, these data support a central role for the MEKK1–JNK cascade in mediating TGF-β/activin signals that control F-actin formation and c-Jun activity, which in turn may regulate epithelium sheet movement during mammalian eyelid closure.
Discussion
Mice homozygous for the Mekk1ΔKD allele, in which the MEKK1 kinase domain is replaced by the bacterial LacZ gene, exhibit an EOB phenotype identical to that of MEKK1-null mutants (Yujiri et al., 2000). The experiments described above explore the molecular mechanisms by which MEKK1 controls eyelid development. We show that MEKK1 is required for actin stress fiber formation and c-Jun N-terminal phosphorylation in the developing eyelid epithelium, most likely resulting from JNK activation by TGF-β/activin. The MEKK1–JNK-mediated TGF-β/ activin signal that controls epidermal keratinocyte migration constitutes the mechanistic basis for the regulation of mouse embryonic eyelid closure.
The initial signal for this pathway is likely derived from a member of the TGF-β superfamily (Hocevar et al., 1999). This signal could, for instance, be generated by activin βB, a member of the TGF-β family whose ablation results in an EOB phenotype similar to that observed in Mekk1ΔKD/ΔKD mice (Vassalli et al., 1994). The homodimer of activin βB subunits, activin B, indeed causes a marked induction of actin stress fiber formation and keratinocyte migration in a MEKK1-dependent fashion. The cell surface receptor for activin βB involved in eyelid development has not been identified, but in keratinocytes, the receptor transducing the activin B signal also appears to be utilized by its close relatives, such as activin A and TGF-β1, which induce similar cell responses.
TGF-β/activin-stimulated JNK activity is completely abolished in MEKK1-deficient cells; in contrast, activation of ERK and nuclear translocation of Smad4 are affected only marginally, if at all, by MEKK1 ablation. Hence, among the many downstream events of TGF-β/activin signaling, only JNK activation requires functional MEKK1. The MEKK1–JNK cascade, although not involved in cell proliferation, is needed for TGF-β/activin-induced actin stress fiber formation and keratinocyte migration, as suppressing JNK activity by a specific chemical inhibitor prevents these cellular activities from taking place, similar to the alterations caused by MEKK1 ablation. Controlling actin stress fiber formation is evidently a downstream effect of the MEKK1–JNK cascade in TGF-β/activin signaling, which may make a major contribution to epithelial cell migration and eyelid closure.
Interestingly, in the ex vivo culture system that we used, keratinocyte migration and actin polymerization in response to TGF-α, another factor crucial for eyelid closure, do not require the MEKK1–JNK pathway, but instead are dependent on the activation of ERK. Therefore we propose that at least two MAPK-dependent pathways are involved in epithelial cell migration: one is the TGF-β/activin-induced MEKK1–JNK pathway and the other is the TGF-α-induced ERK pathway, which may be connected to the c-Jun transcription factor, as discussed later. Activation of either pathway is sufficient for actin stress fiber formation in cultured keratinocytes, but in the context of eyelid development in vivo both activities appear to be required. It is reasonable to suggest that, in addition to their common effects on regulating F-actin, each pathway may control other cell activities essential for eyelid closure.
Another downstream event of the MEKK1–JNK pathway is the induction of c-Jun N-terminal phosphorylation, observed in TGF-β/activin-treated keratinocytes as well as in the developing eyelid epithelium. These results, together with the finding of an EOB phenotype in mice with keratinocyte-specific c-Jun knockout (Li et al., 2003; Zenz et al., 2003), strongly suggest that c-Jun, a well-established target for JNK (Hibi et al., 1993), also makes a contribution to eyelid closure. Despite the involvement of JNK in both events, the enhancement of c-Jun transcriptional activity caused by its N-terminal phosphorylation is unlikely to be directly related to growth-factor-induced actin polymerization because the latter process is very rapid and probably independent of transcription. Therefore we suggest that the MEKK1–JNK cascade has a dual function in the developing eyelid epithelium: on the one hand, it controls actin polymerization; on the other, it causes c-Jun phosphorylation. Most likely, the MEKK1–JNK module, independently of c-Jun, contributes to the initial phase of TGF-β/activin-induced actin polymerization. After the initial signal is delivered, elevated c-Jun transcriptional activity may affect gene expression to enhance the commitment of the eyelid epithelium to the migratory phenotype required for eyelid closure (Figure 7C).
It has been shown by us and others that MEKK1 has a role in the migration of ES cells and MEFs (Yujiri et al., 2000; Xia et al., 2000), but the function of MEKK1 in these cell types does not explain its effects on eyelid closure, a process that is accompanied by increased actin polymerization in the eyelid epithelium. In this regard, lack of actin stress fiber formation and the impairment in keratinocyte migration in response to TGF-β/activin are most likely responsible for the failure in eyelid closure of the Mekk1ΔKD/ΔKD fetuses. By examination of random cell movements, MEKK1 ablation was reported to affect embryonic fibroblast motility (Yujiri et al., 2000). However, we found that dermal fibroblast migration was independent of MEKK1. It is evident that the signals required for random cell motility are not entirely equivalent to those involved in directional cell migration measured by the in vitro wound-healing assay. Never theless, our data strongly indicate that lack of actin fiber formation and impaired c-Jun phosphorylation in the epithelium are the major consequences of MEKK1 ablation.
The Mekk1+/ΔKD mice exhibit normal eyelid closure and eye development, with unperturbed keratinocyte migration and actin polymerization. Therefore expression of the N-terminal regulatory domain by the Mekk1ΔKD allele is unlikely to exert a dominant negative effect on normal wt MEKK1 activity. Nonetheless, in the context of the native MEKK1 protein, the N-terminal regulatory domain, through interaction with the actin cross-linking protein, α-actinin, and with p115 Rho GTPase-activating protein (GAP), may connect MEKK1 to the regulation of cytoskeleton reorganization and actin polymerization (Christerson et al., 2002).
MEKK1 is uniquely important for normal eyelid morphogenesis during embryonic development, an observation that is consistent with its high expression level at the eyelid tips. MEKK1 is also expressed in several other embryonic tissues, such as hair follicles, ears and limbs; however, the normal development and function of these tissues are unaffected by the MEKK1 deficiency. Other members of the MAPKKK subfamily may be acting in these tissues, compensating for the lack of MEKK1. We suggest that the in vivo role of MEKK1, and perhaps of other MAPKKKs, is determined not only by its expression, but also by its uniqueness of function in a specific tissue.
The developmental process of eyelid closure is strikingly similar to Drosophila dorsal closure, in that both involve the movement of epithelium sheets and actin polymerization (Jacinto et al., 2002). Drosophila dorsal closure is controlled by the JNK–AP-1 pathway, which in turn regulates the expression of Drosophila Tgfb that leads to dorsal closure (Sluss et al., 1996; Glise and Noselli, 1997). Our findings suggest that in mammals the MEKK1–JNK pathway acts downstream of TGF-β signaling, controlling F-actin formation and c-Jun phosphorylation in the leading-edge eyelid epithelial cells. Another pathway involved in this process is controlled by the c-Jun-regulated HB-EGF expression and EGFR activation, which may activate the ERK pathway (Li et al., 2003; Zenz et al., 2003). c-Jun transcriptional activation provides the link between the two pathways. The remarkable similarity of the JNK–AP-1 pathway involved in the control of both Drosophila dorsal closure and mammalian eyelid closure suggests an evolutionary conservation in these two processes. Given that EOB is an easily scored phenotype, our findings should be of utility in determining the role of various signaling molecules in activation of the mammalian JNK pathway and other pathways that regulate actin polymerization and in determining the level and specificity of their output. We predict that paralogs of other molecules shown to be involved in dorsal closure in Drosophila will prove to play a similar role in the control of eyelid closure in mammals. Analyzing the functions of such molecules in this system is likely to be instrumental in understanding the molecular organization and evolution of signaling networks across different biological systems.
Materials and methods
Generation of Mekk1ΔKD mice
A targeting vector previously termed MekkllacZ was constructed as described previously (Xia et al., 2000). Two independently targeted ES cells, heterozygous at the Mekk1 locus, lacking the MEKK1 kinase domain (Mekk1+/ΔKD) were used for injection of mouse blastocysts, and several chimeras were crossed with C57BL/6 mice to obtain mice with germline transmission of the Mekk1ΔKD allele. The Mekk1+/ΔKD heterozygous mice were fertile, healthy and appeared normal. All experiments conducted with these animals have been approved by the University of Cincinnati Animal Care and Use Committee.
Histological examination, immunohistochemistry and immunofluorescence staining
E13.5–E18.5 fetuses were fixed in 4% paraformaldehyde, dehydrated with a graded ethanol series and embedded in paraffin. Sections 5 µm thick were deparaffinized by immersing in xylene and rehydrated, before staining with hematoxylin and eosin (H&E) according to standard procedures. The deparaffinized tissue sections were subjected to immunohistochemistry as described (Liu et al., 1993). For immunofluorescent staining, cells grown on 6 mm glass coverslips were treated with MAPK inhibitors and growth factors for various times. Cell fixation, permeabilization and immunostaining were done as described (Nobes and Hall, 1999). Images were obtained using a Nikon Eclips TE-300 microscope.
Whole-mount X-gal staining
Whole-mount X-gal staining of E15.5 fetuses was performed as described (Henkemeyer et al., 1996) and photographed using Olympus DF plan microscope. The stained embryos were processed for embedding, sectioning and X-gal staining. The stained sections were photographed after counterstaining with hematoxylin.
Electron microscopy analyses
Scanning electron microscopy was performed using a Hitachi H-2000 microscope operating at 20 kV as described (Ashrafi et al., 1993). Transmission electron microscopy was done as previously described (Birk and Mayne, 1997). Sections were examined and photographed at 75 kV using a Hitachi 7000 transmission electron microscope.
Cells, reagents and antibodies
Primary mouse epidermal keratinocytes and dermal fibroblasts were prepared from Mekk1+/ΔKD or Mekk1ΔKD/ΔKD neonatal mice. The Mekk1ΔKD/ΔKD pups displayed EOB phenotype and their genotypes were confirmed by established PCR methods. Keratinocytes were prepared as described (Rouabhia et al., 1992) and all cell culture reagents were from Invitrogen. FITC–phalloidin, 4′-6-diamidine-2-phenylindole (DAPI) and 5-bromo-2-deoxyuridine (BrdU) were from Sigma, TGF-α and TGF-β1 were from PeproTech, and activin A and activin B from R&D Systems Inc. Antibodies for BrdU were from Jackson Research Laboratories, those for c-Jun, phospho-c-Jun (S63) and Smad4 were from Santa Cruz Biotechnology, those for phospho-JNK, ERK and p38 were from Promega and those for total JNK, ERK and p38 were from Pharmingen. The kinase inhibitors PD98059 and SP600125 were from Calbiochem.
In vitro wound-healing assay
Confluent monolayers of Mekk1+/ΔKD and Mekk1ΔKD/ΔKD epidermal keratinocytes and dermal fibroblasts were starved in growth-factor-free media for 24 h before being wounded by scratching the monolayer with a micropipette tip. The cells were washed, the wounded cells were grown at 37 °C in appropriate media with or without growth factors as indicated and the wounded area was photographed immediately and 24 h after wounding.
RNA isolation, reverse transcription and real-time quantitative polymerase chain reaction
Total RNA was isolated from primary cultured Mekk1+/ΔKD and Mekk1ΔKD/ΔKD keratinocytes using Tri-reagent (Molecular Research Center) and purified by RNeasy Mini Kit (QIAGEN). Reverse transcription was performed using SuperScript II RNase H– reverse transcriptase (Invitrogen).
Real-time PCR was carried out with Cepheid PCR Analizer using SYBR® Green I (Invitrogen) as the detection format. The reactions were cycled 35 times under the appropriate parameters for each pair of primers and the fluorescence was measured every 15 s at the end of each cycle to construct the amplification curve. All determinations were performed at least in triplicate. The primers were as follows: TGF-βR-I: 5′-AACCGCACTGTCATTCACC-3′ (forward), 5′-CGCCAAACTTCTCCAAACC-3′ (reverse); TGF-βR-II: 5′-AAGGAAAAGAAAAGGGCGG–3′ (forward), 5′-GGACACGGTAGCAGTAGAAG-3′ (reverse); ActR-I: 5′-ACACCAAAACCACCCTAACC-3′(forward), 5′-CGAAAGATACGCAGAGAGCC–3′ (reverse); ActR-IB: 5′-GAC ACC ATA GAC ATT GCT CC-3′ (forward), 5′-CATACAACCTTTCGCATCTCC-3′ (reverse); ActR-II: 5′-CAC AGC CCA CTT CAA ATC C-3′ (forward), 5′-GACACAACCAAATCTTCCCC-3′ (reverse); ActR-IIB: 5′-TCGATGAGTACATGCTGCC-3′ (forward), 5′-GTGTTTCAGCCAGTGATCC-3′ (reverse).
BrdU incorporation
Cell proliferation was determined by measuring incorporation of BrdU as described (Morrison et al., 1999). BrdU-positive cells were counted under fluorescence microscopy. Standard deviation was calculated.
Laser scanning confocal microscopy
In situ staining for F-actin was carried out using eye samples of E15.5 embryos fixed in 4% paraformaldehye. Tissues were incubated with FITC–phalloidin (Molecular Probe Inc.) and propidium iodide and observed by laser scanning confocal microscopy (Leica, SP2 Multiphoton Microscope) as previously described (Petroll et al., 2001).
Western blot analyses
Confluent monolayers of Mekk1+/ΔKD and Mekk1ΔKD/ΔKD epidermal keratinocytes were treated with growth factors for various times and the cell lysates were prepared as described (Hibi et al., 1993). Protein lysates (100 µg) were separated by SDS–PAGE, transferred onto nitrocellulose membrane and probed with antibodies for phosphorylated JNK, ERK and p38, and for total JNK, ERK and p38.
Acknowledgments
Acknowledgements
We thank Dr Alvaro Puga for a critical reading of the manuscript, Dr Yinling Hu for helping with keratinocyte culture, Yong Ma for statistical analyses, and Biao Zuo and Mingya Huang for technical assistance. This work is supported in part by NIH grants P30 ES06096 (Y.X.), EY 11845 and EY 13755 (W.W.-Y.K.), EY 12486 (C.Y.L.), EY05129 (D.E.B.), ES04151 and ES06376 (M.K.), and ACS 92–026–09 (Y.X.), Ohio Cancer Research Associates (Y.X.), and unrestricted grants from Research to Prevent Blindness (RPB), New York, NY and Ohio Lions Eye Research Foundation, Columbus, OH. W.W.-Y.K. is the recipient of RPB’s Senior Scientific Investigator Award.
References
- Ashrafi S.H., Atassi,B., Erickson,R. and Sabet,T. (1993) Migration of epithelium during phenytoin-dependent gingival overgrowth in mice. Scanning Microsc., 7, 1247–1253. [PubMed] [Google Scholar]
- Bakin A.V., Tomlinson,A.K., Bhowmick,N.A., Moses,H.L. and Arteaga,C.L. (2000) Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem., 275, 36803–36810. [DOI] [PubMed] [Google Scholar]
- Beck P.L., Rosenberg,I.M., Xavier,R.J., Koh,T., Wong,J.F. and Podolsky,D.K. (2003) transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am. J. Pathol., 162, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz E.A. et al. (1996) Characterization of the mouse transforming growth factor alpha gene: its expression during eyelid development and in waved 1 tissues. Cell Growth Differ., 7, 1271–1282. [PubMed] [Google Scholar]
- Birk D.E. and Mayne,R. (1997) Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol., 72, 352–361. [PubMed] [Google Scholar]
- Christerson L.B., Gallagher,E., Vanderbilt,C.A., Whitehurst,A.W., Wells,C., Kazempour,R., Sternweis,P.C. and Cobb,M.H. (2002) p115 Rho GTPase activating protein interacts with MEKK1. J. Cell Physiol., 192, 200–208. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Findlater G.S., McDougall,R.D. and Kaufman,M.H. (1993) Eyelid development, fusion and subsequent reopening in the mouse. J. Anat., 183, 121–129. [PMC free article] [PubMed] [Google Scholar]
- Gibson E.M., Henson,E.S., Villanueva,J. and Gibson,S.B. (2002) MEK kinase 1 induces mitochondrial permeability transition leading to apoptosis independent of cytochrome c release. J. Biol. Chem., 277, 10573–10580. [DOI] [PubMed] [Google Scholar]
- Glise B. and Noselli,S. (1997) Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev., 11, 1738–1747. [DOI] [PubMed] [Google Scholar]
- Han Z., Boyle,D.L., Chang,L., Bennett,B., Karin,M., Yang,L., Manning,A.M. and Firestein,G.S. (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest, 108, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M.J. and Juriloff,D.M. (1986) Eyelid development and fusion induced by cortisone treatment in mutant, lidgap-Miller, fetal mice. A scanning electron microscope study. J. Embryol. Exp. Morphol., 91, 1–18. [PubMed] [Google Scholar]
- Henkemeyer M., Orioli,D., Henderson,J.T., Saxton,T.M., Roder,J., Pawson,T. and Klein,R. (1996) Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell, 86, 35–46. [DOI] [PubMed] [Google Scholar]
- Hibi M., Lin,A., Smeal,T., Minden,A. and Karin,M. (1993) Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev., 7, 2135–2148. [DOI] [PubMed] [Google Scholar]
- Hocevar B.A., Brown,T.L. and Howe,P.H. (1999) TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J., 18, 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto A., Woolner,S. and Martin,P. (2002) Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev. Cell, 3, 9–19. [DOI] [PubMed] [Google Scholar]
- Karin M. (1996) The regulation of AP-1 activity by mitogen-activated protein kinases. Phil. Trans. R. Soc. Lond. B Biol. Sci., 351, 127–134. [DOI] [PubMed] [Google Scholar]
- Kim K.Y., Jeong,S.Y., Won,J., Ryu,P.D. and Nam,M.J. (2001) Induction of angiogenesis by expression of soluble type II transforming growth factor-beta receptor in mouse hepatoma. J. Biol. Chem., 276, 38781–38786. [DOI] [PubMed] [Google Scholar]
- Kuan C.Y., Yang,D.D., Samanta Roy,D.R., Davis,R.J., Rakic,P. and Flavell,R.A. (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron, 22, 667–676. [DOI] [PubMed] [Google Scholar]
- Kultz D., Madhany,S. and Burg,M.B. (1998) Hyperosmolality causes growth arrest of murine kidney cells. Induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J. Biol. Chem., 273, 13645–13651. [DOI] [PubMed] [Google Scholar]
- Li G., Gustafson-Brown,C., Hanks,S.K., Nason,K., Arbeit,J.M., Pogliano,K., Wisdom,R.M. and Johnson,R.S. (2003) c-Jun is essential for organization of the epidermal leading edge. Dev. Cell, 4, 865–877. [DOI] [PubMed] [Google Scholar]
- Lin A., Minden,A., Martinetto,H., Claret,F.X., Lange-Carter,C., Mercurio,F., Johnson,G.L. and Karin,M. (1995) Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science, 268, 286–290. [DOI] [PubMed] [Google Scholar]
- Liu C.Y., Zhu,G., Westerhausen-Larson,A., Converse,R., Kao,C.W., Sun,T.T. and Kao,W.W. (1993) Cornea-specific expression of K12 keratin during mouse development. Curr. Eye Res., 12, 963–974. [DOI] [PubMed] [Google Scholar]
- Minamino T., Yujiri,T., Terada,N., Taffet,G.E., Michael,L.H., Johnson,G.L. and Schneider,M.D. (2002) MEKK1 is essential for cardiac hypertrophy and dysfunction induced by Gq. Proc. Natl Acad. Sci. USA, 99, 3866–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., White,P.M., Zock,C. and Anderson,D.J. (1999) Prospective identification, isolation by flow cytometry and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell, 96, 737–749. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1999) Rho GTPases control polarity, protrusion and adhesion during cell movement. J. Cell Biol., 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll W.M., Ma,L., Jester,J.V., Cavanagh,H.D. and Bean,J. (2001) Organization of junctional proteins in proliferating cat corneal endothelium during wound healing. Cornea, 20, 73–80. [DOI] [PubMed] [Google Scholar]
- Rouabhia M., Germain,L., Belanger,F., Guignard,R. and Auger,F.A. (1992) Optimization of murine keratinocyte culture for the production of graftable epidermal sheets. J. Dermatol., 19, 325–334. [DOI] [PubMed] [Google Scholar]
- Sluss H.K. and Davis,R.J. (1997) Embryonic morphogenesis signaling pathway mediated by JNK targets the transcription factor JUN and the TGF-beta homologue Decapentaplegic. J. Cell. Biochem., 67, 1–12. [PubMed] [Google Scholar]
- Sluss H.K., Han,Z., Barrett,T., Davis,R.J. and Ip,Y.T. (1996) A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev., 10, 2745–2758. [DOI] [PubMed] [Google Scholar]
- Tao L., Wadsworth,S., Mercer,J., Mueller,C., Lynn,K., Siekierka,J. and August,A. (2002) Opposing roles of serine/threonine kinases MEKK1 and LOK in regulating the CD28 responsive element in T-cells. Biochem. J., 363, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C., Thomas,G., Pierre,J., Jacquemin,C., Pierre,M. and Saunier,B. (1997) Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase). Eur. J. Biochem., 244, 587–595. [DOI] [PubMed] [Google Scholar]
- Vassalli A., Matzuk,M.M., Gardner,H.A., Lee,K.F. and Jaenisch,R. (1994). Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev., 8, 414–427. [DOI] [PubMed] [Google Scholar]
- Vuong H., Patterson,T., Shapiro,P., Kalvakolanu,D.V., Wu,R., Ma,W.Y., Dong,Z., Kleeberger,S.R. and Reddy,S.P. (2000). Phorbol ester-induced expression of airway squamous cell differentiation marker, SPRR1B, is regulated by protein kinase Cdelta/Ras/MEKK1/MKK1-dependent/AP-1 signal transduction pathway. J. Biol. Chem., 275, 32250–32259. [DOI] [PubMed] [Google Scholar]
- Wu Z., Wu,J., Jacinto,E. and Karin,M. (1997). Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol. Cell. Biol., 17, 7407–7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Wu,Z., Su,B., Murray,B. and Karin,M. (1998). JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev., 12, 3369–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Makris,C., Su,B., Li,E., Yang,J., Nemerow,G.R. and Karin,M. (2000). MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl Acad. Sci. USA, 97, 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Dai,T., Deak,J.C., Kyriakis,J.M., Zon,L.I., Woodgett,J.R. and Templeton,D.J. (1994). Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature, 372, 798–800. [DOI] [PubMed] [Google Scholar]
- Yujiri T., Sather,S., Fanger,G.R. and Johnson,G.L. (1998). Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science, 282, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Yujiri T. et al. (2000). MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc. Natl Acad. Sci. USA, 97, 7272–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenz R., Scheuch,H., Martin,P., Frank,C., Eferl,R., Kenner,L., Sibilia,M. and Wagner,E.F. (2003). c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev. Cell, 4, 879–889. [DOI] [PubMed] [Google Scholar]