Introduction
To acknowledge the 50th anniversary of the (IFN) discovery of interferon, a number of excellent reviews have been written on various aspects of the IFN system. However only a few of us remember, that at the beginning, IFN and its multiple mechanisms of action were regarded with suspicion. Here was a protein that could not be purified and could be detected only by its function and these were many. It is not surprising that in the years that preceded the cloning and isolation of this biological modifier, the general opinion was that most IFN-induced effects were artifacts. It was only with the development of recombinant technologies and availability of genetically modified mouse models that the IFN genes could be characterized and the molecular basis of interferon induction and action began to be established.
We know now that type I interferons (IFNα and IFNβ) are group of related proteins with antiviral, antiproliferative, and immunomodulating activities that are essential components of the innate antiviral response (1). These proteins are induced by viral infection or by activation of Toll like receptors (TLR) 3, 4, 7 and 9 upon binding of their respective ligands (2). The biological response to IFN requires binding of IFN to cell type specific receptors, which results in their dimerization and activation of downstream signaling by non-receptor tyrosine kinases Janus (JAK) and phosphorylation of signal transducers and activators of transcription (STAT1 and STAT2 proteins). The phosphorylation of STAT proteins results in their homo and heterodimerization, nuclear translocation, and binding to the interferon response elements in the promoters of interferon stimulated genes (ISG) (3). The IFN induced JAK-STAT pathway activates transcription of hundreds of ISGs (4) and while some of the proteins encoded by these genes have a direct antiviral activity (1), (5), (6), the functions of most of the ISGs have not yet been characterized. However it is anticipated that many of them participate in the innate antiviral response either directly or indirectly.
One of the first recognized ISGs was ISG15 (7), (8). This gene is induced not only by Type I IFN and viral infection (9), (10), but also by IRF-3, treatment with LPS, retinoic acid, campothecin and following radiation (11), (10). Expression of ISG15 has been detected in many inducible transcriptional profiles as well, indicating that this gene is induced in response to diverse stress stimuli. ISG15 was the first identified member of the family of ubiquitin-like proteins, which has grown to include additional protein modifiers with appreciable sequence homology to ubiquitin, such as SUMO, Nedd8, and Fat 10, among others (12). Despite the relatively early discovery of ISG15 as a protein modifier, 30 years elapsed before the identification of first targets of ISG15 conjugation. Presently about 200 ISGylated proteins of different functions have been identified. However how ISGylation affects their cellular distribution and function is yet not clear (13), (14), (15)
Our interest in ISG15 was initiated by observations that implicated a role for ISG15 in the retinoic acid (RA) therapeutic response in acute myeleocytic leukemia (AML) (16). Subsequently learning that ISG15 is a ubiquitin like protein, we hypothesized that ISG15 might interfere with the ubiquitination of cellular and viral proteins. In this review we will focus on the possible role of ISG15 in the general antiviral response and in the regulation of cell growth and carcinogenesis.
ISG15
ISG15 is expressed as an inactive, precursor protein that is cleaved by specific proteases to expose the carboxyl-terminal diglycine motif that is central to protein conjugation (17). Recently a crystal structure of ISG15 was determined (18). Covalent attachment of Ub to proteins employs a cascade of reactions that requires multiple enzymes (19), (20). ISG15 conjugation is accomplished through a similar set of reactions that utilize enzymes unique to ISG15 conjugation as well as enzymes involved in Ub conjugation. The activating enzyme of the ISG15 conjugating pathway is the Ub-activating E1-like protein (UBE1L). Expression of UBE1L can be stimulated by IFN (21) and by certain/specific retinoids in acute promyelocytic leukemia cells (22). Two E2 enzymes implicated in the Ub conjugation cascade, UbcH8 and UbcH6, are also E2 enzyme for ISG15 conjugation. Intersection of the ubiquitin and ISG15 conjugation pathways appears to occur with ISG15 E3 enzymes as well. The ISG15 E3 enzymes Epf and Herc5 have each been also implicated in ubiquitin conjugation (23), (24). Epf was shown to target 14-3-3α for ISG15 conjugation as well as Ub conjugation. HerC5 was shown to regulate global ISG15 conjugation and exhibited in vitro Ub ligase activity. However conjugation of ISG15 to intracellular target proteins was shown to be distinct from that targeted by Ub (25, 26).
Both protein ubiquitination and ISGylation are reversible by deconjugating enzymes. While multiple proteins were shown to have Ub deconjugating activity, as reviewed (27), only one protein, UBP43, has exhibited ISG15 deconjugation activity in vitro (26). UBP43 knockout mice have elevated levels of conjugated ISG15 (28), and siRNA-mediated knockdown of UBP43 mRNA led to increased ISG15 conjugation following IFN treatment. Interestingly, type I IFN treatment was shown to induce also expression of enzymes that lead to ISG15 conjugation (UBE1L, Ubch8, HerC5, Efp) with concurrent induction of UBP43 (29), (21). Co-ordinate induction of ISG15 conjugating and de-conjugation enzymes by IFN indicates that the levels of conjugated ISG15 during the innate antiviral response are regulated by ISG15 conjugation and deconjugation (28).
Ub-mediated proteolysis plays an important role in cell cycle regulation, modulation of the transcription factors activities, antigen presentation and in the regulation of the immune and inflammatory responses (30). The large versatility and specificity of this system is given by a large number of the Ub specific ligases-E3. In general, poly-ubiquitination of lysine 43 targets proteins towards proteasome-dependent degradation, mono-ubiquitination is a signal for internalization and vesicles sorting, and poly-ubiquitinaiton through lysine 63 is a signal for kinases activation (31). Many viruses harness the Ub–proteasome pathway to alter cellular signaling and antiviral response. For example, HSV-1 encoded ICP0 is an E3 like Ub ligase (32), (33), and KSHV immediate-early transcription factor RTA encodes Ub E3 ligase activity that targets IRF-7 degradation (34). HPV and adenovirus exploit the cellular ubiquitinating system to stimulate Ub-mediated degradation of growth regulatory genes p53 and Rb (35), (36), (37).
While the degradation by proteosome generally depends on prior Ub conjugation, protein modification by ISG15 does not typically cause substrate degradation (38), instead it may alter the subcellular localization, structure, stability, or activity of targeted proteins (39 ). Large number of cellular proteins that are associated with cellular skeleton, stress response and chromatin remodeling were identified as ISG15 targets. ISG15 also targets proteins that play a role in the innate antiviral response, including: PKR, MxA, Stat1, Jak1 and Rig I (13), (40). How the ISGylation affects function of these proteins is largely unknown.
The role of ISGylation pathway in the antiviral response
ISG15 is one of the earliest ISG induced by type I IFN and ISG15 conjugation was shown to target many components of the antiviral innate signalling pathway (40) (21). These results suggested that ISG15 may play a role in the antiviral innate immune response. However the functional consequences of ISG15 conjugation in modulating this response are largely unknown.
Two observations have indicated that ISG15 may play a role in the innate immune response. The first was in influenza infection. The NS1 protein of influenza B virus inhibited ISGylation, indicating a possible antiviral role for ISG15 in influenza B virus infection (21). The second was the finding that infection with the parasite Theileria annulata down-regulated expression of ISG15 and Ubp43 and that elevated levels of ISG15 were observed upon the parasite death. The mechanism by which Theileia infection down regulates ISGylation is not yet known. However these results indicate that this parasite down regulates ISGylation pathway to circumvent the innate host response that would limit is replication (41).
The results of the studies on the molecular mechanism by which ISG15 modulates the antiviral response have been inconsistent. Although, it was initially suggested that ISGylation plays an important role in the regulation of JAK-STAT pathway and IFN signaling (42), (26), (28), no evidence of a defect in IFN signaling was observed in ISG15 null mice (43) or mice defective in ISG15 activating enzyme UBE1L (44). The evidence for the direct antiviral role of ISG15 has not been clearly established and the in vivo analysis gave some contradictory results. While on one hand the replication of the Sinbis virus-expressing ISG15 was attenuated in IFNRα1 deficient mice (45), and the ISG15 null mice have shown an increase susceptibility to Sinbis virus, influenza virus and HSV-1 infection (46), there was no difference between the replication of VSV and LCMV in ISG15 null and wt mice (43, 47). These studies suggest that the antiviral effect of ISG15 may be virus specific, however, the mechanism by which ISG15 mediates its antiviral effect has not been addressed in these studies.
ISG15 targets ubiquitination steps in HIV-1 assembly
We and others have shown some years ago the type I IFN inhibits retroviral and lentiviral replication in vitro and that IFN-mediated inhibition targets virus assembly (48), (49) (49). Since the assembly of HIV-1 depends on ubiquination of the Gag polyprotein, we hypothesized that IFN-mediated inhibition was mediated by ISG15 interference with Gag ubiquitination. We found that expression of ectopic ISG15 mimicked the antiviral effect of IFN and that the inhibition of ISG15 expression in interferon treated cells by ISG15 specific siRNA reversed IFN inhibition and rescued HIV-1 release (50), (51). Further investigation into the molecular mechanisms of this inhibition revealed that ISG15 inhibits the ubiquitination steps that are required for virus release. Ectopic ISG15 inhibited ubiquitination of the Gag polypeptide and consequently interaction of the p6 domain of the Gag polyprotein with Tsg101, a central component of the endosomal sorting complex (ESCRT-1) (52). The amino-terminal domain of Tsg101 ( UEV motif), which has homology to E2 enzymes (53) binds to Ub and this interaction is essential for the targeting of Ub conjugated proteins to multi-vesicular bodies (MVB) (54) However, Tsg101 UEV motif binds also to the P(T/S)AP motif in the L domain of HIV-1 Gag polypeptide and the crystal structure analysis revealed that the UEV domain can bind both Ub and PTAP peptide simultaneously (55). Interestingly, HIV-1 release is inhibited also in the presence of proteasome inhibitors indicating that a non-specific down regulation of proteosome-mediated protein degradation is also inhibitory (56).
The precise mechanism by which ISG15 interferes with the HIV-1 Vps pathway is not yet clear, since direct ISG15 conjugation to Gag or Tsg101 could not be demonstrated. However, there are additional mechanisms by which ISG15 conjugation can inhibit ubiquitination. The ubiquitin E2 enzyme Ubc13 was shown to be a target of ISG15 conjugation and ISGylation of Ubc13 suppressed its ability to form thioester intermediates with Ub (57), which lead to inhibition of K-63 linked ubiquitination. Thus the inhibition of Gag or Tsg101 ubiquitination may be a consequence of ISGylation of Ubc13. The ISG15 mediated inhibition of viral assembly is not limited to HIV-1 and extends to the assembly of viruses that also use the ubiquitin dependant endosomal pathway for its assembly (Okumura et al unpublished). Unfortunately the role of ISG15 on HIV-1 replication could not be tested in ISG15 null mice and the mechanism by which ISG15 effects replication of Sinbis, influenza and HSV-1 virus has not been yet addressed. However it should be noted that HSV-1 encoded immediate early IPC0 protein is an E3 ubiquin ligase suggesting the E3 mediated ubiquitination could be targeted by ISG15.
ISG15 subverts the Ub mediated degradation of IRF-3 in infected cells
The effect of ISG15 in viral replication can be also indirect. Virally induced ISG15 conjugation promotes the antiviral state by subverting proteasome-mediated degradation of IRF-3 in infected cells. We have shown that IRF-3 is ubiquitinated in infected cells and ISG15 inhibits ubiquitination and ubiquitin-mediated degradation of IRF-3. Thus the expression of ISG15 in infected cells slows IRF-3 degradation, facilitates nuclear translocation of IRF-3, and stimulates IRF-3 mediated induction of IFNβ promoter (58). It was shown recently that ubiquitin-mediated degradation of IRF-3 is regulated by Pin1, which binds IRF-3, however, Pin1 itself does not catalyze IRF-3 ubiquitination or proteasome degradation (59). Phosphorylated IRF-3 is also recognized by E3 ubiquitin ligase – cullin1 resulting in degradation of IRF-3 in the nucleus (60). Whether Pin1, IRF-3 and cullin1 are components of the ubiquitin protein ligase complex (SCF), formation of which is inhibited by ISG15 is yet to be determined. The observation that ISG15 counteracts the down regulation of IRF-3 transcriptional activity through proteasome degradation points to the existence of an antiviral loop in which ISG15 induction in virus infected cells by activated IRF-3 and IFN, stabilizes IRF-3 and protect its degradation by Ub pathway.
The role of Ubp43
It should be noted that the ISG15 isopeptidase Ubp43 was shown to regulate negatively the interferon response. Induction of ISGs and ISGylation was strongly enhanced in macrophages deficient in Ubp43 and UBP43 null mice were more resistant to viral and microbial infections than the wt mice. This effect was originally attributed to the observation that ISGylation of STAT1 prolonged STAT1 phosphorylation and it was suggested that ISG15 conjugation was an important mediator of IFN signalling (61). However it was shown later, that Ubp43 down modulation of type I IFN signalling pathway was independent of the ISG15 isopeptidase activity of UBP43, but was rather due to the binding of Ubp43 to the subunit of Type I IFN receptor (R2) and consequent interference with binding of JAK1 to IFNR2. Thus Ubp43 can regulate the antiviral response both by down regulation the ISG15 conjugation and as a part of the negative feedback loop of IFN signalling pathway (62)
The role of ISG15 in carcinogenesis and cancer therapy
The IFNs have shown clinical efficacy, as single agent therapy and in combination therapy, for the treatment of multiple hematologic and solid malignancies, as reviewed (63, 64). Use of IFN in cancer therapy, however, has been limited by significant toxicity with dose escalation and by resistance to therapy. Opportunities for continued progress exist within these limitations. IFNs exert anticancer effects through regulation of 100s of genes and diverse cellular pathways that control cell-cycle progression, apoptosis, and immune surveillance, among other functions. An in-depth understanding of these pathways and the roles played by IFN-regulated proteins holds the potential to (1) increase the efficacy of IFN therapy, (2) lead to the development of more targeted cancer therapies, (3) lead to the use of novel drug combinations, (4) or develop strategies for reducing drug toxicity. Here, we examine the role of IFN-induced ISG15 and the proteins involved in the ISG15 conjugation pathway in carcinogenesis and cancer therapy.
ISG15
In addition to being a post-translational protein modifier ISG15 is also a secreted cytokine. Each of these roles has been implicated in carcinogenesis. As a secreted protein, ISG15 was shown to modulate immune cell activation. Secreted ISG15 isolated from the media of melanoma cell lines activated monocyte-derived dendritic cells (DC) in vitro (65). Neutralization of ISG15 in the media from these cell lines with ISG15-specific antibodies inhibited induction of e-cadherin, a marker of DC activation. These results suggest that melanoma cells might secrete ISG15 to influence surrounding immune cells and perhaps to evade immune surveillance by altering DC migration. Other studies have examined expression of intracellular ISG15 in tumor samples. Intracellular ISG15 expression was increased in bladder cancers when compared to normal bladder tissue (66). Increased ISG15 expression in bladder cancers positively correlated with the degree of tumor invasion and was independent of bladder inflammation as determined by pathologic examination as well as urine nitrate and leukocyte esterase measurement. Interestingly, on western analysis, only the unconjugated form of ISG15 was detected and immunohistochemistry revealed that ISG15 is located in the nuclei of cancer cells. Heterogeneous levels of ISG15 conjugation were observed among numerous breast cancer cell lines and tumor samples from diverse tissues when compared to adjacent normal tissue (67). In breast cancer cell lines tested, ISG15 expression was inversely proportional to polyubiquitination. Forced expression of ISG15 was shown to decrease ubiquitination and knockdown of ISG15 with siRNA led to increased ubiquitination. Taken together these data demonstrated an inverse correlation between ISG15 conjugation and ubiquitination in certain cancers.
UBE1L
The gene for UBE1L is located on the short arm of chromosome 3 (3p21), which consistently shows loss of heterozygosity (LOH) in numerous human cancers including small cell lung carcinoma (SCLC) (68) and renal cell carcinoma, among others. The frequent deletion of this chromosomal region in cancers led to the tempting hypothesis that genes in 3p21, and more specifically UBE1L, are potential tumor suppressors. Prior to identification of UBE1L as the activating enzyme for ISG15, it was shown that while UBE1L expression was present in normal lung tissue and lung cell lines it was not detectable in 14 lung cancer cell lines tested (69). More recent testing demonstrated reduced levels of UBE1L protein in lung cancer tissue when compared to adjacent normal tissue (22). Interestingly, loss of UBE1L expression in SCLC cell lines was not due to loss of the second UBE1L allele because small amounts of UBE1L mRNA were detected in these cell lines (70).
The effect of altered UBE1L expression on ISG15 conjugation has been demonstrated in several settings. Knock down of UBE1L mRNA with specific small-inhibitory RNAs decreased retinoid as well as interferon induced ISG15 conjugation (16). In addition, introduction of UBE1L to K562 cells, a hematopoietic cancer cell line lacking UBE1L expression, restored interferon-induced ISG15 conjugation and activation of reporters containing IREs (61). These results suggest that reduced or absent UBE1L expression in cancer may dramatically inhibit ISG15 conjugation in these cancers and, in turn, significantly modulate IFN signaling within these cancers. Further investigations into the role of ISG15 conjugation in IFN signaling and the effects of reduced UBE1L expression on ISG15 conjugation in tumor samples are required. To date, investigations have shown increased ISG15 expression and conjugation in tumor samples. Given the frequent loss of 3p21 in some tumors and the effects of reduced UBE1L expression on ISG15 conjugation, one would anticipate reduced ISG15 conjugation in these tumors. Whether this hypothesis proves accurate in actual tumor biopsies, remains to be determined.
Our interest in the ISG15ylation pathway was initiated by observations that UBE1L expression and ISG15 conjugation were induced during all-trans-retinoic acid (ATRA)-mediated differentiation of NB4 acute promyelocytic leukemia (APL) cells (11, 16). Adenoviral mediated introduction of UBE1L into this cell line caused apoptosis and cotransfection of UBE1L with the oncoprotein PML/RAR∞ decreased levels of PML/RAR∞ - a marker of therapeutic response to retinoids in APL (11). Regulation of this pathway following ATRA treatment was encountered again in a cancer chemoprevention model in which chronic ATRA treatment prevented bronchial epithelial cell transformation by the NNK – a known carcinogen found in tobacco smoke (71). ISG15 and UBE1L expression was elevated in an in vitro model of retinoid-mediated lung cancer chemoprevention (22). Cyclin D1 levels were implicated previously as a marker of retinoid response and as a retinoid target (72, 73). An inverse relationship between cyclin D1 levels and UBE1L expression was observed in human lung tumor biopsies and adjacent normal tissue. Co-transfection of UBE1L with cyclin D1 in vitro resulted in decreased levels of cyclin D1. These results implicated the importance of the ISG15 conjugation pathway in retinoid-based chemotherapy and cancer chemoprevention. At least two mechanisms may contribute to this effect: (1) introduction of UBE1L in the NB4 acute promyelocytic leukemia line induces cellular death via apoptosis suggesting that enhancement of ISG15 conjugation could play a role in cancer therapy, and (2) UBE1L may influence the stability of the PML/RAR∞ oncoprotein and the cell cycle regulator cyclin D1. It has been demonstrated previously that ATRA treatment of NB4 APL cells induces IFN secretion; therefore the finding that ATRA induced type I IFN secretion may contribute to ATRA-induced ISG15 conjugation is not altogether surprising (74). Retinoids employ diverse signaling pathways in exerting effects on cellular systems and organisms. These observations implicate the recruitment of ISG15 conjugation in retinoid-induced differentiation therapy and cancer chemoprevention (11, 16).
E2 and E3 enzymes
While the role of ISG15’s E2 and E3 enzymes in cancer has yet to be explored fully, a compelling role for the ISG15 E3 ligase enzyme Efp in cancer has developed. Efp is a target gene of the ERα receptor that has been implicated as a mediator of estrogen-dependent growth regulation. Efp disrupted mice have underdeveloped uteri and reduced estrogen responsiveness (75). Antisense mediated knockdown of Efp expression in MCF7 cells reduced estrogen-dependent tumor growth in athymic mice and Efp over expression increased tumor size in this same model (76). Efp is also an ISG (23, 77). Interestingly, Efp can act as an E3 enzyme for both ubiquitin and ISG15. As an ubiquitin ligase, Efp targets 14-3-3α - a negative regulator of cell cycle progression - for proteolysis (76). As an ISG15 ligase, Efp targets 14-3-3α and itself for ISG15 conjugation. SiRNA mediated knockdown of Efp inhibited type I IFN-induced conjugation of ISG15 to 14-3-3α (78). Efp also mediates self-ISG15 conjugation (77, 79). Expression of an Efp mutant that is resistant to self-ISG15 conjugation enhanced ISG15 conjugation to 14-3-3α suggesting that self-ISG15 conjugation acts as a negative regulator of Efp activity (79).
Analysis of tumor samples largely supported the hypothesized role of Efp as an estrogen target gene involved in down regulation of 14-3-3α. Immunohistochemical analysis of endometrial adenocarcinomas and normal endometrial tissue demonstrated increased Efp and estrogen receptor (ER) expression in endometrial adenocarcinomas (80). Increased Efp in cancer cells was inversely proportional with 14-3-3α levels in these samples. In ovarian carcinomas there was significant correlation between Efp and ER expression and Efp expression was significantly increased in patients with advanced disease (81). However, expression of Efp in breast carcinomas has yielded conflicting results. One recent study demonstrated that Efp levels correlated positively with ER status and lymph node status but correlated negatively with 14-3-3α levels (82). In contrast, previous studies noted no correlation between Efp and ER status in breast tumor biopsies (83) and show no significant change in Efp levels in early stages of breast carcinogenesis, despite demonstrated decrease in 14-3-3α levels (84).
The E2 and E3 enzymes appear to be sites of interactions between the ubiquitin conjugation system and ISG15 conjugation. Ubch8, Efp, and HerC5 were implicated as having roles in both ISG15 and ubiquitin conjugation. In addition, E2 and E3 enzymes of the ubiquitin and ISG15 conjugation pathways are targets of modification with Ub and ISG15 – another potential site of crosstalk between these pathways. For example, ISG15ylation of Ubch13, an E2 enzyme in the ubiquitin conjugation system, inhibits its ability to form a thioester linkage with ubiquitin (85, 86), suggesting that ISG15ylation of this enzyme might negatively inhibit its role in ubiquitin conjugation.
The role of the interaction between these two pathways in carcinogenesis and cancer therapy is intriguing for several reasons. The ubiquitin conjugation pathway has been implicated in diverse cellular processes and alterations in this pathway are present in multiple human cancers. Inhibition of ubiquitin-dependent degradation has proven an effective chemotherapeutic approach for multiple cancers. The proteasome inhibitor bortezomib is clinically effective in treating multiple myeloma and shows promise in the treatment of additional malignancies, as reviewed (87, 88). Understanding more fully the mechanisms of communication between these two pathways and the role that this communication plays in carcinogenesis could provide valuable insight into carcinogenesis and lead to novel therapeutic advances.
UBP43
The role of this type I IFN-induced, multifunctional protein in cancer has not been yet clearly established. As described previously, UBP43 acts as an ISG15 isopeptidase, removing conjugated ISG15 from target proteins. UBP43 also negatively regulated the type I IFN signaling pathway by a mechanism that is independent of its ISG15 isopeptidase activity (62). Although cloned originally from AML-ETO knockin mice – a mouse model of CML – and human melanoma cell lines treated with IFNβ, there is no additional evidence of altered UBP43 expression in human cancer samples. A mechanism for UBP43 down-regulation was discovered and this mechanism does support the hypothesis that UBP43 protein levels could be diminished in tumors. UBP43 was shown to be a target of Skp2-dependent proteolysis (89). Skp2 −/− mice exhibit elevated levels of UBP43 and diminished ISG15 conjugation. Cotransfection of Skp2 and UBP43 led to proteasome-dependent UBP43 degradation and enhanced UBP43 poly-ubiquitination. Skp2 is a member of the F-box family of proteins that function as substrate recognition factors for SCF E3-ligase complexes. Skp2 was implicated previously in the ubiquitin-dependent degradation of the regulators of cell proliferation p27, p57, p130, and cyclin E, as reviewed (90). An inverse correlation between levels of Skp2 and p27 protein has been demonstrated in multiple human cancers. In some human tumors this inverse correlation parallels tumor grade. The relationship between Skp2 levels and UBP43 levels in human tumors and the functional implications of Skp2-dependent UBP43 degradation on cell cycle and cancer progression remains to be determined.
The role of UBP43 as a negative regulator of ISG15 conjugation and IFN signaling suggests that UBP43 inhibition could be a valid target for future cancer therapy (16, 91). The therapeutic potential of UBP43 inhibition was highlighted recently by utilizing a mouse model of BRL-ABL driven chronic myelogenous leukemia (CML) – a leukemia that is responsive to IFN therapy (91). There was increased latency of BCR-ABL driven leukemogenesis in mice transplanted with UBP43 −/− bone marrow cells when compared to BCR-ABL-induced leukemogenesis in normal, wild-type mice. This latency required IFN signaling as double knockout mice with deletion of both UBP43 and IFNRA1 developed leukemia in a similar manner to control mice.
Reflections and perspectives
Recent studies established that type I IFNs regulate diverse cellular functions in addition to the critical role they play in the innate, antiviral response. While restriction of pathogen invasion by controlling viral life cycle and cellular death is an essential part of the innate immune response, exposure to type I IFN modulates the expression of a surprisingly large number of genes. A question then arises: does the profound multiplicity of type I IFN-regulated genes reflect multiple avenues of defense from pathogens, or do IFN-regulated genes play roles independent of pathogen defense? Findings that have implicated ISGs not only in the cellular response to pathogen invasion and pathogen-induced stress, but also in cellular growth and carcinogenesis suggest that both possibilities are true. Thus, a further understanding of the cellular functions of ISGs has the potential to reveal basic mechanisms underlying the cellular response to pathogen infection as well as other non-infectious pathologies such as cancer.
We hoped to demonstrate the diversity of ISG function across infectious and non-infectious pathologies by examining the role of the IFN-induced, ubiquitin-like protein ISG15 in viral defense and cancer. While, for many years, this ISG was used only as a marker of type I IFN signaling, recent studies have revealed mechanisms of ISG15 conjugation and a signaling role for secreted ISG15. One fascinating aspect of ISG15 conjugation is its interactions with the ubiquitin conjugation pathway. Studies demonstrated a role for ISG15 in cellular defense from infections as well as a possible role for the ISG15 in carcinogenesis and cancer therapy. Finally as therapeutic strategies become more targeted, it seems that future clinical therapy might increase therapeutic effectiveness and decrease toxicity by modulating specific ISGs rather than relying on use of type I IFNs.
Fig. 1.
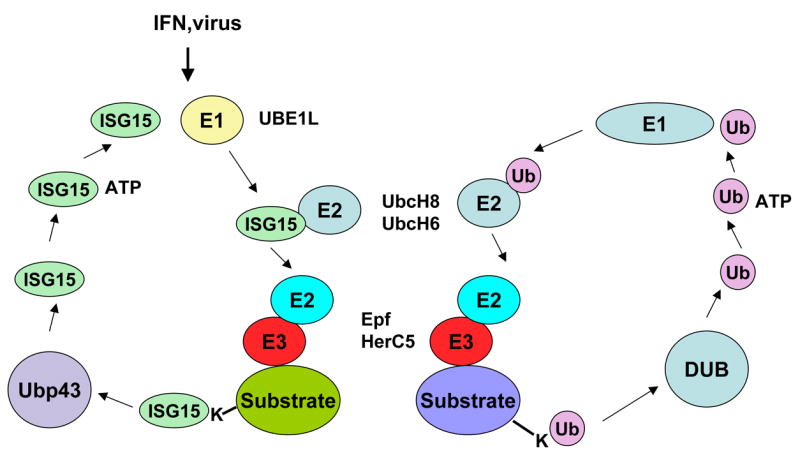
Similarities between enzymatic pathways leading to the conjugation of ubiquitin and ISG15 to cellular proteins.
Acknowledgments
We thank to Drs. E. Dmitrovsky for his mentorship and support and A. Okumura for helpful discussion on the covered subjects. PMP laboratory was supported by NIAID grant R01 AI19737-19 and AI054537.
Biographies
 Ian F. Pitha-Rowe graduated Magna Cum Laude from Haverford College where he received BS in chemistry. While in Haverford he was recipient of Howard Hughes junior fellowship. He is currently enrolled in the MD/PhD program at Dartmouth Medical School. He obtained his PhD in Pharmacology in 2004. During the graduate studies his research was focused on “Post-Translational Modification in Retinoid Action: A Role for UBE1L in Cancer Therapy and Chemoprevention.” He is completing his clinical training and will graduate from medical school this year.
Ian F. Pitha-Rowe graduated Magna Cum Laude from Haverford College where he received BS in chemistry. While in Haverford he was recipient of Howard Hughes junior fellowship. He is currently enrolled in the MD/PhD program at Dartmouth Medical School. He obtained his PhD in Pharmacology in 2004. During the graduate studies his research was focused on “Post-Translational Modification in Retinoid Action: A Role for UBE1L in Cancer Therapy and Chemoprevention.” He is completing his clinical training and will graduate from medical school this year.
 Paula M. Pitha is professor of Oncology and Molecular Biology and Genetics at the Johns Hopkins School of Medicine. She obtained her PhD in Biochemistry from The Czechoslovak Academy of Sciences in Prague and her postdoctoral training at the National Research Council in Ottawa, Canada and the Curie Institute in Paris, France. Since joining the Johns Hopkins faculty in 1971 she has focus her research on molecular mechanism of the induction of type I interferon and the role of interferon in retroviral and lentiviral infections. She was Leukemia Society Scholar and recipient of Eleanor Roosevelt Fellowship. For her contribution to the understanding of the innate antiviral responses on molecular levels she has received the Milstein Award in 1996 and the Gregory Mendel Award in Biological Sciences in 2004. She was Advisor of the NIAAIDC council, member of Advisory board of FDA and Basic Science Subcommittee on AIDS, NIH, and NAAIDC. Currently she is chair of the Advisory committee, VirxSys corporation, Gaithersburg, MD.
Paula M. Pitha is professor of Oncology and Molecular Biology and Genetics at the Johns Hopkins School of Medicine. She obtained her PhD in Biochemistry from The Czechoslovak Academy of Sciences in Prague and her postdoctoral training at the National Research Council in Ottawa, Canada and the Curie Institute in Paris, France. Since joining the Johns Hopkins faculty in 1971 she has focus her research on molecular mechanism of the induction of type I interferon and the role of interferon in retroviral and lentiviral infections. She was Leukemia Society Scholar and recipient of Eleanor Roosevelt Fellowship. For her contribution to the understanding of the innate antiviral responses on molecular levels she has received the Milstein Award in 1996 and the Gregory Mendel Award in Biological Sciences in 2004. She was Advisor of the NIAAIDC council, member of Advisory board of FDA and Basic Science Subcommittee on AIDS, NIH, and NAAIDC. Currently she is chair of the Advisory committee, VirxSys corporation, Gaithersburg, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Paun A, Pitha PM. Biochimie. 2007. The IRF family, revisited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 4.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 5.Sen GC, Ranshoff RM. Interferon-induced antiviral actions and their regulation. Adv Virus Res. 1993;42:57–102. doi: 10.1016/s0065-3527(08)60083-4. [DOI] [PubMed] [Google Scholar]
- 6.Martensen PM, Justesen J. Small ISGs coming forward. J Interferon Cytokine Res. 2004;24:1–19. doi: 10.1089/107999004772719864. [DOI] [PubMed] [Google Scholar]
- 7.Farrell PJ, Broeze RJ, Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979;279:523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- 8.Blomstrom DC, Fahey D, Kutny R, Korant BD, Knight E., Jr Molecular characterization of the interferon-induced 15-kDa protein. Molecular cloning nucleotide and amino acid sequence. J Biol Chem. 1986;261:8811–8816. [PubMed] [Google Scholar]
- 9.Reich N, Evans B, Levy D, Fahey DE, Knight J, Darnell JEJ. Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci USA. 1987;84:6394–6398. doi: 10.1073/pnas.84.18.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas AL, Ahrens P, Bright PM, Ankel H. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J Biol Chem. 1987;262:11315–11323. [PubMed] [Google Scholar]
- 11.Kitareewan S, Pitha-Rowe I, Sekula D, Lowrey CH, Nemeth MJ, Golub TR, Freemantle SJ, Dmitrovsky E. UBE1L is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:3806–3811. doi: 10.1073/pnas.052011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochstrasser M. Evolution and function of ubiquitin-like protein-conjugation systems. Nat Cell Biol. 2000;2:E153–157. doi: 10.1038/35019643. [DOI] [PubMed] [Google Scholar]
- 13.Malakhov MP, Kim KI, Malakhova OA, Jacobs BS, Borden EC, Zhang DE. High-throughput immunoblotting. Ubiquitiin-like protein ISG15 modifies key regulators of signal transduction. J Biol Chem. 2003;278:16608–16613. doi: 10.1074/jbc.M208435200. [DOI] [PubMed] [Google Scholar]
- 14.Giannakopoulos NV, Luo JK, Papov V, Zou W, Lenschow DJ, Jacobs BS, Borden EC, Li J, Virgin HW, Zhang DE. Proteomic identification of proteins conjugated to ISG15 in mouse and human cells. Biochem Biophys Res Commun. 2005;336:496–506. doi: 10.1016/j.bbrc.2005.08.132. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci U S A. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitha-Rowe I, Hassel BA, Dmitrovsky E. Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia. J Biol Chem. 2004;279:18178–18187. doi: 10.1074/jbc.M309259200. [DOI] [PubMed] [Google Scholar]
- 17.Jentsch S, Pyrowolakis G. Ubiquitin and its kin: how close are the family ties? Trends Cell Biol. 2000;10:335–342. doi: 10.1016/s0962-8924(00)01785-2. [DOI] [PubMed] [Google Scholar]
- 18.Narasimhan J, Wang M, Fu Z, Klein JM, Haas AA, Kim JJ. Crystal structure of the interferon-induced ubiquitin-like protein ISG15. J Biol Chem. 2005 doi: 10.1074/jbc.M502814200. [DOI] [PubMed] [Google Scholar]
- 19.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Staub O. Ubiquitylation and isgylation: overlapping enzymatic cascades do the job. Sci STKE. 2004;2004:pe43. doi: 10.1126/stke.2452004pe43. [DOI] [PubMed] [Google Scholar]
- 21.Yuan W, Krug RM. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. Embo J. 2001;20:362–371. doi: 10.1093/emboj/20.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitha-Rowe I, Petty WJ, Feng Q, Koza-Taylor PH, Dimattia DA, Pinder L, Dragnev KH, Memoli N, Memoli V, Turi T, Beebe J, Kitareewan S, Dmitrovsky E. Microarray analyses uncover UBE1L as a candidate target gene for lung cancer chemoprevention. Cancer Res. 2004;64:8109–8115. doi: 10.1158/0008-5472.CAN-03-3938. [DOI] [PubMed] [Google Scholar]
- 23.Zou W, Zhang DE. The interferon-inducible ubiquitin-protein isopeptide ligase (E3) EFP also functions as an ISG15 E3 ligase. J Biol Chem. 2006;281:3989–3994. doi: 10.1074/jbc.M510787200. [DOI] [PubMed] [Google Scholar]
- 24.Horie-Inoue K, Inoue S. Epigenetic and proteolytic inactivation of 14-3-3sigma in breast and prostate cancers. Semin Cancer Biol. 2006;16:235–239. doi: 10.1016/j.semcancer.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Narasimhan J, Potter JL, Haas AL. Conjugation of the 15-kDa interferon-induced ubiquitin homolog is distinct from that of ubiquitin. J Biol Chem. 1996;271:324–330. doi: 10.1074/jbc.271.1.324. [DOI] [PubMed] [Google Scholar]
- 26.Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem. 2002;277:9976–9981. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. Faseb J. 1997;11:1245–1256. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- 28.Ritchie KJ, Malakhov MP, Hetherington CJ, Zhou L, Little MT, Malakhova OA, Sipe JC, Orkin SH, Zhang DE. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev. 2002;16:2207–2212. doi: 10.1101/gad.1010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyman TA, Matikainen S, Sareneva T, Julkunen I, Kalkkinen N. Proteome analysis reveals ubiquitin-conjugating enzymes to be a new family of interferon-alpha-regulated genes. Eur J Biochem. 2000;267:4011–4019. doi: 10.1046/j.1432-1327.2000.01433.x. [DOI] [PubMed] [Google Scholar]
- 30.Ciechanover A. The ubiquitin-mediated proteolytic pathway: mechanisms of action and cellular physiology. Biol Chem Hoppe Seyler. 1994;375:565–581. doi: 10.1515/bchm3.1994.375.9.565. [DOI] [PubMed] [Google Scholar]
- 31.Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 32.Boutell C, Sadis S, Everett RD. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J Virol. 2002;76:841–850. doi: 10.1128/JVI.76.2.841-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155:1265–1273. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Y, Wang SE, Hayward GS. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22:59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Talis AL, Huibregtse JM, Howley PM. The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV)-positive and HPV-negative cells. J Biol Chem. 1998;273:6439–6445. doi: 10.1074/jbc.273.11.6439. [DOI] [PubMed] [Google Scholar]
- 36.Helt AM, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- 37.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- 38.Liu M, Li XL, Hassel BA. Proteasomes modulate conjugation to the ubiquitin-like protein, ISG15. J Biol Chem. 2003;278:1594–1602. doi: 10.1074/jbc.M208123200. [DOI] [PubMed] [Google Scholar]
- 39.Yeh ET, Gong L, Kamitani T. Ubiquitin-like proteins: new wines in new bottles. Gene. 2000;248:1–14. doi: 10.1016/s0378-1119(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oura CA, McKellar S, Swan DG, Okan E, Shiels BR. Infection of bovine cells by the protozoan parasite Theileria annulata modulates expression of the ISGylation system. Cell Microbiol. 2006;8:276–288. doi: 10.1111/j.1462-5822.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- 42.Malakhova O, Malakhov M, Hetherington C, Zhang DE. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J Biol Chem. 2002;277:14703–14711. doi: 10.1074/jbc.M111527200. [DOI] [PubMed] [Google Scholar]
- 43.Osiak A, Utermohlen O, Niendorf S, Horak I, Knobeloch KP. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol Cell Biol. 2005;25:6338–6345. doi: 10.1128/MCB.25.15.6338-6345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim KI, Yan M, Malakhova O, Luo JK, Shen MF, Zou W, de la Torre JC, Zhang DE. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Mol Cell Biol. 2006;26:472–479. doi: 10.1128/MCB.26.2.472-479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenschow DJ, Giannakopoulos NV, Gunn LJ, Johnston C, O’Guin AK, Schmidt RE, Levine B, Virgin HWt. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79:13974–13983. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenschow DJ, Lai C, Frias-Staheli N, Giannakopoulos NV, Lutz A, Wolff T, Osiak A, Levine B, Schmidt RE, Garcia-Sastre A, Leib DA, Pekosz A, Knobeloch KP, Horak I, Virgin HWt. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci U S A. 2007;104:1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knobeloch KP, Utermohlen O, Kisser A, Prinz M, Horak I. Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice. Mol Cell Biol. 2005;25:11030–11034. doi: 10.1128/MCB.25.24.11030-11034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gendelman HE, Baca LM, Turpin J, Kalter DC, Hansen B, Orenstein JM, Dieffenbach CW, Friedman RM, Meltzer MS. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol. 1990;145:2669–2676. [PubMed] [Google Scholar]
- 49.Popik W, Pitha PM. Exploitation of cellular signaling by HIV-1: unwelcome guests with master keys that signal their entry. Virology. 2000;276:1–6. doi: 10.1006/viro.2000.0581. [DOI] [PubMed] [Google Scholar]
- 50.Kunzi MS, Pitha PM. Role of interferon-stimulated gene ISG-15 in the interferon-omega-mediated inhibition of human immunodeficiency virus replication. J Interferon Cytokine Res. 1996;16:919–927. doi: 10.1089/jir.1996.16.919. [DOI] [PubMed] [Google Scholar]
- 51.Okumura A, Lu G, Pitha-Rowe I, Pitha PM. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci U S A. 2006;103:1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bishop N, Woodman P. TSG101/mammalian VPS23 and mammalian VPS28 interact directly and are recruited to VPS4-induced endosomes. J Biol Chem. 2001;276:11735–11742. doi: 10.1074/jbc.M009863200. [DOI] [PubMed] [Google Scholar]
- 53.Ponting CP, Cai YD, Bork P. The breast cancer gene product TSG101: a regulator of ubiquitination? J Mol Med. 1997;75:467–469. [PubMed] [Google Scholar]
- 54.Bilodeau PS, Winistorfer SC, Kearney WR, Robertson AD, Piper RC. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J Cell Biol. 2003;163:237–243. doi: 10.1083/jcb.200305007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundquist WI, Schubert HL, Kelly BN, Hill GC, Holton JM, Hill CP. Ubiquitin recognition by the human TSG101 protein. Mol Cell. 2004;13:783–789. doi: 10.1016/s1097-2765(04)00129-7. [DOI] [PubMed] [Google Scholar]
- 56.Schubert U, Ott DE, Chertova EN, Welker R, Tessmer U, Princiotta MF, Bennink JR, Krausslich HG, Yewdell JW. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci U S A. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi T, Yokosawa H. ISG15 modification of Ubc13 suppresses its ubiquitin-conjugating activity. Biochem Biophys Res Commun. 2005 doi: 10.1016/j.bbrc.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 58.Lu G, Reinert JT, Pitha-Rowe I, Okumura A, Kellum M, Knobeloch KP, Hassel B, Pitha PM. ISG15 enhances the innate antiviral response by inhibition of IRF-3 degradation. Cell Mol Biol (Noisy-le-grand) 2006;52:29–41. [PubMed] [Google Scholar]
- 59.Saitoh T, Tun-Kyi A, Ryo A, Yamamoto M, Finn G, Fujita T, Akira S, Yamamoto N, Lu KP, Yamaoka S. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol. 2006;7:598–605. doi: 10.1038/ni1347. [DOI] [PubMed] [Google Scholar]
- 60.Bibeau-Poirier A, Gravel SP, Clement JF, Rolland S, Rodier G, Coulombe P, Hiscott J, Grandvaux N, Meloche S, Servant MJ. Involvement of the IkappaB kinase (IKK)-related kinases tank-binding kinase 1/IKKi and cullin-based ubiquitin ligases in IFN regulatory factor-3 degradation. J Immunol. 2006;177:5059–5067. doi: 10.4049/jimmunol.177.8.5059. [DOI] [PubMed] [Google Scholar]
- 61.Malakhova OA, Yan M, Malakhov MP, Yuan Y, Ritchie KJ, Kim KI, Peterson LF, Shuai K, Zhang DE. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17:455–460. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malakhova OA, Kim KI, Luo JK, Zou W, Kumar KG, Fuchs SY, Shuai K, Zhang DE. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. Embo J. 2006;25:2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol. 2000;10:125–144. doi: 10.1006/scbi.2000.0315. [DOI] [PubMed] [Google Scholar]
- 64.Vannucchi S, Chiantore MV, Mangino G, Percario ZA, Affabris E, Fiorucci G, Romeo G. Perspectives in biomolecular therapeutic intervention in cancer: from the early to the new strategies with type I interferons. Curr Med Chem. 2007;14:667–679. doi: 10.2174/092986707780059616. [DOI] [PubMed] [Google Scholar]
- 65.Padovan E, Terracciano L, Certa U, Jacobs B, Reschner A, Bolli M, Spagnoli GC, Borden EC, Heberer M. Interferon stimulated gene 15 constitutively produced by melanoma cells induces e-cadherin expression on human dendritic cells. Cancer Res. 2002;62:3453–3458. [PubMed] [Google Scholar]
- 66.Andersen JB, Aaboe M, Borden EC, Goloubeva OG, Hassel BA, Orntoft TF. Stage-associated overexpression of the ubiquitin-like protein, ISG15, in bladder cancer. Br J Cancer. 2006;94:1465–1471. doi: 10.1038/sj.bjc.6603099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desai SD, Haas AL, Wood LM, Tsai YC, Pestka S, Rubin EH, Saleem A, Nur EKA, Liu LF. Elevated expression of ISG15 in tumor cells interferes with the ubiquitin/26S proteasome pathway. Cancer Res. 2006;66:921–928. doi: 10.1158/0008-5472.CAN-05-1123. [DOI] [PubMed] [Google Scholar]
- 68.Pitterle DM, Jolicoeur EM, Bepler G. Hot spots for molecular genetic alterations in lung cancer. In Vivo. 1998;12:643–658. [PubMed] [Google Scholar]
- 69.McLaughlin PM, Helfrich W, Kok K, Mulder M, Hu SW, Brinker MG, Ruiters MH, de Leij LF, Buys CH. The ubiquitin-activating enzyme E1-like protein in lung cancer cell lines. Int J Cancer. 2000;85:871–876. doi: 10.1002/(sici)1097-0215(20000315)85:6<871::aid-ijc22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 70.Carritt B, Kok K, van den Berg A, Osinga J, Pilz A, Hofstra RM, Davis MB, van der Veen AY, Rabbitts PH, Gulati K, et al. Cancer Res. 1992;52:1536–1541. [PubMed] [Google Scholar]
- 71.Langenfeld J, Lonardo F, Kiyokawa H, Passalaris T, Ahn MJ, Rusch V, Dmitrovsky E. Inhibited transformation of immortalized human bronchial epithelial cells by retinoic acid is linked to cyclin E down-regulation. Oncogene. 1996;13:1983–1990. [PubMed] [Google Scholar]
- 72.Boyle JO, Langenfeld J, Lonardo F, Sekula D, Reczek P, Rusch V, Dawson MI, Dmitrovsky E. Cyclin D1 proteolysis: a retinoid chemoprevention signal in normal, immortalized, and transformed human bronchial epithelial cells. J Natl Cancer Inst. 1999;91:373–379. doi: 10.1093/jnci/91.4.373. [DOI] [PubMed] [Google Scholar]
- 73.Dragnev KH, Freemantle SJ, Spinella MJ, Dmitrovsky E. Cyclin proteolysis as a retinoid cancer prevention mechanism. Ann N Y Acad Sci. 2001;952:13–22. doi: 10.1111/j.1749-6632.2001.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 74.Dao CT, Luo JK, Zhang DE. Retinoic acid-induced protein ISGylation is dependent on interferon signal transduction. Blood Cells Mol Dis. 2006;36:406–413. doi: 10.1016/j.bcmd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Orimo A, Inoue S, Minowa O, Tominaga N, Tomioka Y, Sato M, Kuno J, Hiroi H, Shimizu Y, Suzuki M, Noda T, Muramatsu M. Underdeveloped uterus and reduced estrogen responsiveness in mice with disruption of the estrogen-responsive finger protein gene, which is a direct target of estrogen receptor alpha. Proc Natl Acad Sci U S A. 1999;96:12027–12032. doi: 10.1073/pnas.96.21.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urano T, Saito T, Tsukui T, Fujita M, Hosoi T, Muramatsu M, Ouchi Y, Inoue S. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature. 2002;417:871–875. doi: 10.1038/nature00826. [DOI] [PubMed] [Google Scholar]
- 77.Nakasato N, Ikeda K, Urano T, Horie-Inoue K, Takeda S, Inoue S. A ubiquitin E3 ligase Efp is up-regulated by interferons and conjugated with ISG15. Biochem Biophys Res Commun. 2006;351:540–546. doi: 10.1016/j.bbrc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 78.Takeuchi T, Inoue S, Yokosawa H. Identification and Herc5-mediated ISGylation of novel target proteins. Biochem Biophys Res Commun. 2006;348:473–477. doi: 10.1016/j.bbrc.2006.07.076. [DOI] [PubMed] [Google Scholar]
- 79.Zou W, Wang J, Zhang DE. Negative regulation of ISG15 E3 ligase EFP through its autoISGylation. Biochem Biophys Res Commun. 2007;354:321–327. doi: 10.1016/j.bbrc.2006.12.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakayama H, Sano T, Motegi A, Oyama T, Nakajima T. Increasing 14-3-3 sigma expression with declining estrogen receptor alpha and estrogen-responsive finger protein expression defines malignant progression of endometrial carcinoma. Pathol Int. 2005;55:707–715. doi: 10.1111/j.1440-1827.2005.01900.x. [DOI] [PubMed] [Google Scholar]
- 81.Sakuma M, Akahira J, Suzuki T, Inoue S, Ito K, Moriya T, Sasano H, Okamura K, Yaegashi N. Expression of estrogen-responsive finger protein (Efp) is associated with advanced disease in human epithelial ovarian cancer. Gynecol Oncol. 2005;99:664–670. doi: 10.1016/j.ygyno.2005.07.103. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki T, Urano T, Tsukui T, Horie-Inoue K, Moriya T, Ishida T, Muramatsu M, Ouchi Y, Sasano H, Inoue S. Estrogen-responsive finger protein as a new potential biomarker for breast cancer. Clin Cancer Res. 2005;11:6148–6154. doi: 10.1158/1078-0432.CCR-05-0040. [DOI] [PubMed] [Google Scholar]
- 83.Thomson SD, Ali S, Pickles L, Taylor J, Pace PE, Lymboura M, Shousha S, Coombes RC. Analysis of estrogen-responsive finger protein expression in benign and malignant human breast. Int J Cancer. 2001;91:152–158. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1032>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 84.Simooka H, Oyama T, Sano T, Horiguchi J, Nakajima T. Immunohistochemical analysis of 14-3-3 sigma and related proteins in hyperplastic and neoplastic breast lesions, with particular reference to early carcinogenesis. Pathol Int. 2004;54:595–602. doi: 10.1111/j.1440-1827.2004.01668.x. [DOI] [PubMed] [Google Scholar]
- 85.Takeuchi T, Yokosawa H. ISG15 modification of Ubc13 suppresses its ubiquitin-conjugating activity. Biochem Biophys Res Commun. 2005;336:9–13. doi: 10.1016/j.bbrc.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 86.Zou W, Papov V, Malakhova O, Kim KI, Dao C, Li J, Zhang DE. ISG15 modification of ubiquitin E2 Ubc13 disrupts its ability to form thioester bond with ubiquitin. Biochem Biophys Res Commun. 2005;336:61–68. doi: 10.1016/j.bbrc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 87.Roccaro AM, Hideshima T, Richardson PG, Russo D, Ribatti D, Vacca A, Dammacco F, Anderson KC. Bortezomib as an antitumor agent. Curr Pharm Biotechnol. 2006;7:441–448. doi: 10.2174/138920106779116865. [DOI] [PubMed] [Google Scholar]
- 88.Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P. Proteasome inhibitors: antitumor effects and beyond. Leukemia. 2007;21:30–36. doi: 10.1038/sj.leu.2404444. [DOI] [PubMed] [Google Scholar]
- 89.Tokarz S, Berset C, La Rue J, Friedman K, Nakayama K, Nakayama K, Zhang DE, Lanker S. The ISG15 isopeptidase UBP43 is regulated by proteolysis via the SCFSkp2 ubiquitin ligase. J Biol Chem. 2004;279:46424–46430. doi: 10.1074/jbc.M403189200. [DOI] [PubMed] [Google Scholar]
- 90.Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 91.Yan M, Luo JK, Ritchie KJ, Sakai I, Takeuchi K, Ren R, Zhang DE. Ubp43 regulates BCR-ABL leukemogenesis via the Type I interferon receptor signaling. Blood. 2007 doi: 10.1182/blood-2006-07-033209. [DOI] [PMC free article] [PubMed] [Google Scholar]


