Abstract
Background: Surgical resection is the best established treatment known to provide long-term survival and possibility of cure for liver malignancy. Intraoperative blood loss has been the major concern during major liver resections, and mortality and morbidity of surgery are clearly associated with the amount of blood loss. Different techniques have been developed to minimize intraoperative blood loss during liver resection. The radiofrequency ablation (RFA) technique has been used widely in the treatment of unresectable liver tumors. This review concentrates on the use of RFA to provide an avascular liver resection plane. Methods and results: The following review is based on two types of RFA device during liver resection: single needle probe RFA and the In-Line RFA device. Conclusion: Liver resection assisted by RFA is safe and is associated with very limited blood loss.
Keywords: Blood loss, RFA-assisted liver resection, In-Line device
Introduction
Both primary and metastatic cancers of the liver are common and lethal diseases. In Western countries, primary liver cancer is comparatively rare, but is rapidly increasing due to the increased prevalence of hepatitis C, and metastatic colorectal carcinoma is the major indication for liver resection 1,2,3. Primary liver cancer is, however, much more common in other parts of the world, representing 10–50% of malignancies in Africa and parts of Asia 4,5,6.
Currently, surgical resection is the optimal treatment known to provide long-term survival and possibility of cure for both primary and metastatic liver cancer 7,8,9. Unfortunately, intraoperative blood loss has been a major concern during major liver resections, and mortality and morbidity are clearly associated with the amount of blood loss 10,11. The mean blood loss has been reported to be between 600 ml and 1300 ml 12,13, with 28–47% of the patients requiring blood transfusion 12,14. Several studies have shown that blood loss correlates adversely with length of hospital stay, complication rate, and patient survival 15,16. It also has been shown that patients requiring more than 10 units of blood after liver resection for colorectal cancer metastasis have an increased risk of tumor recurrence and poor survival, probably due to immunosuppression 17,18.
Therefore, over the past decade many techniques have been developed to minimize intraoperative blood loss during liver resection 19,20,21,22. Surgeons can decrease intraoperative blood loss by using hypotensive anesthesia to reduce the central venous pressure 23, Pringle's maneuver 24, or total vascular exclusion 25. Parenchymal transection can be performed with the finger fracture or Kelly clamp/crush, using the ultrasonic dissector 20, the WaterJet dissector 26, or stapling devices 27.
Radiofrequency ablation (RFA) has been broadly used for unsectable liver tumors and produces coagulative necrosis of the liver parenchyma and thrombosis and coagulation of small blood vessels 28,29,30,31,32,33. Several recent studies have shown that the use of RFA to assist liver resection decreased blood loss dramatically 34,35,36,37,38,39,40,41,42,43,44. This new technique employs the heat produced by the radiofrequency device to coagulate the liver tissue before resecting it. A plane of tissue is coagulated by the RFA device, after which resection is performed along the coagulated tissue plane. By using this technique, blood loss has significantly decreased to a mean of 30 ml 38.
Currently, there are two types of RFA device used in liver resection: single needle probe RFA and In-Line RFA. Both were successfully used in animal and patient experiments to reduce blood loss in liver surgery. This review will attempt to characterize the unique clinical features of RFA-assisted liver resection.
Methods
Single needle probe RFA
RFA-assisted liver resections using a single needle probe have been done in different medical centres (Table I). All patients underwent careful preoperative assessment for their disease, including spiral computed tomography (CT) scanning or magnetic resonance imaging (MRI), and showed no evidence of unresectable extrahepatic disease. Surgical resections ranged from multiple metastasectomies to bisegmentectomies.
Table I. Clinical details of RFA-assisted liver resection.
| First author | Medical center, city, country | Year | No. | Age (years) | Types of resection | Operation time (min) | Blood loss | Transfusion | Complication | Hospital stay (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Navarra 34 | Department of Human Pathology, University of Messina, Messina, Italy | 01/2001–01/2002 | 27 | Open hepatectomy | 47.5 (30–110) | 30 ml (15–992) | Nil | Nil | 8 (5–86) | |
| Tepel 36 | Klinik fur Allgemeine Chirurgie und Thoraxchirurgie, Universitatsklinikum Schleswig-Holstein, Campus Kiel, Germany | 7 | 74 (41–78) | 1 segment (2); 2 segments (3); > 2 segment (2) | 1 case | Second degree burn to the thigh (1); bile leak (1) | ||||
| Navarra 37 | Department of Surgical Oncology and Technology, Imperial College School of Medicine, Hammersmith Hospital Campus, London, UK | 01/2001–07/2002 | 42 | 57.5 (25–79) | 1 segment (13); 2 segments (16); > 2 segment (13) | 50 (30–110) | 30 ml (15–992) | Nil | Subphrenic abscess (1); chest infection (1); biliary leak (1) | |
| Weber 38 | Department of Surgical Oncology and Technology, Faculty of Medicine, Imperial College of Science, Technology and Medicine, Hammersmith Hospital Campus, London, UK | 01/2000–06/2001 | 15 | 59 (36–75) | 1 segment (8); 2 segments (6); > 2 segments (1) | 205 (95–300) | 30±10 ml | Nil | Nil | 8 (5–9) |
| Beppu 39 | Department of Gastroenterological Surgery, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan | 2004 | 7 | 64 | Laparoscopic hepatectomy | 256 | 96 g | Nil | Nil | 11 |
| Weber 40 | Centre de Chirurgie Viscerale et Transplantation, Hopitaux Universitaires de Strasbourg, Avenue Moliere, Strasbourg, France | 2002 | 1 | 43 | Laparoscopic hepatectomy | 300 | 75 ml | Nil | Nil | 6 |
| Stella 42 | Department of Surgery, Santa Corona Hospital, Pietra Ligure, Savona, Italy | 06/2002–11/2002 | 8 | 64.8 (55–72) | 1 segment (3); 2 segments (3); > 2 segments (2) | 220 (170–420) | 46 ml (5–150) | Nil | Abscess (1) | 9.4 (5–20) |
| Navarra 43 | Department of Surgical Oncology and Technology, Faculty of Medicine, Imperial College of Science, Technology and Medicine, Hammersmith Hospital Campus, London, UK | 2002 | 1 | Right hepatectomy | 80 | 30 ml | Nil | Nil | 9 |
Liver resections were carried out under general anesthesia. The peritoneal cavity was examined for evidence of extrahepatic disease. After dividing intra-abdominal adhesions and the falciform ligament, the liver was then mobilized according to the size and site of the lesion to be resected. An intraoperative ultrasonogram was always performed prior to liver resection.
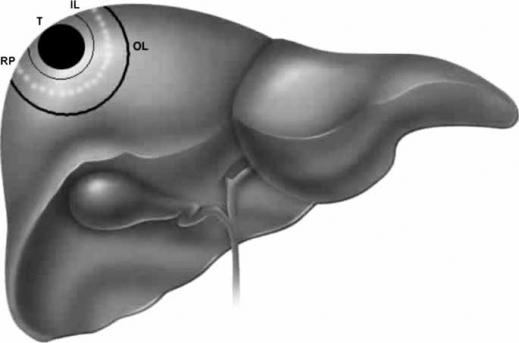
Habib's group has previously described the technique of RFA-assisted liver resection 38. In step 1 (Figure 1), an inner line is made on the liver surface with diathermy to mark the periphery of the tumor, assisted with bimanual palpation and intraoperative ultrasound. It is important to do this first, because after RFA is used, the parenchyma is hardened and it becomes difficult to feel the tumor edge. Also, after RFA, it is impossible to visualize the tumor edge with intraoperative ultrasound; this is a real and important difference which must be understood with all RFA-assisted liver resections.
Figure 1. .
Single needle probe RFA-assisted liver resection. T, tumor; IL, inner line; OL, outer line; RP, resection plane.
In step 2, an outer line, again using diathermy, is made on the liver capsule 2 cm outside (away from) the inner line to mark the site where the probe is going to achieve coagulative necrosis.
In step 3, coagulative necrosis is produced along the outer line using a RFA probe, whilst Habib's group have used the saline-cooled RFA probe; essentially all ablation devices can be used for this purpose.
In step 4, further probe applications are deployed to obtain a zone of necrosis according to the depth of the liver parenchyma to be resected. Each application of RFA energy will need to be applied for about 60 s. The surgeon should check that each probe is correctly positioned by means of ultrasound.
In step 5, the liver parenchyma is transected by using the scalpel. The plane of division should be situated midway between the inner and outer line so as to leave a 1 cm resection margin away from the tumor and leave in situ 1 cm of burned coagulated surface.
All of these groups achieved very good results 34,36,37,38,39,40,42,43 (Table I). Habib et al. demonstrated that blood loss could be reduced to <30±10 ml for a right hepatectomy, and practically zero blood loss on most occasions 38. In all of their 108 patients, only one case needed blood transfusion 36. Other complications included: one second degree burn to the thigh 36, two bile leaks 36,37, one subphrenic abscess 37, and one chest infection 37. These complications were from two studies 36,37. Patients in other studies underwent an uneventful recovery; no significant bile leak, abscess, or requirement for transfusion was encountered.
In-Line RFA
The In-Line RFA device was developed by our group 35,41. The principle of the In-Line RFA device is that a very precise RFA area is created between electrodes of alternating polarity leading to localized heating and coagulation of tissue including blood vessels up to approximately 3 mm. This transection plane is then dissected almost bloodlessly and the larger vessels/biliary structures are tied, sewn or otherwise secured.
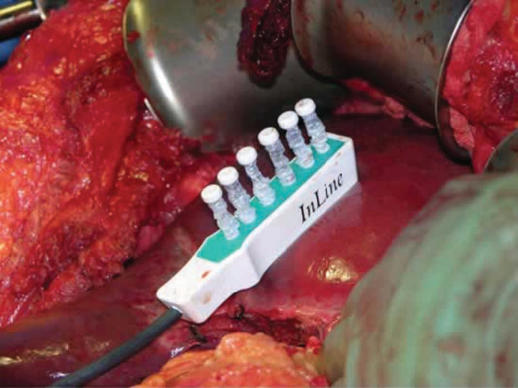
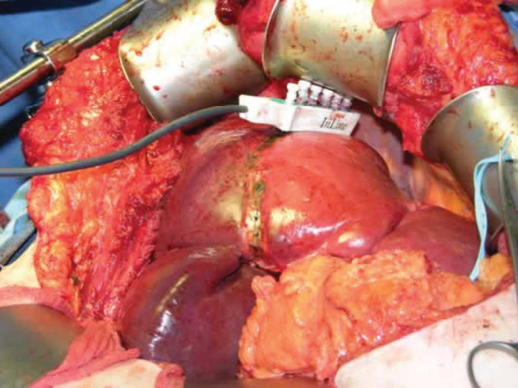
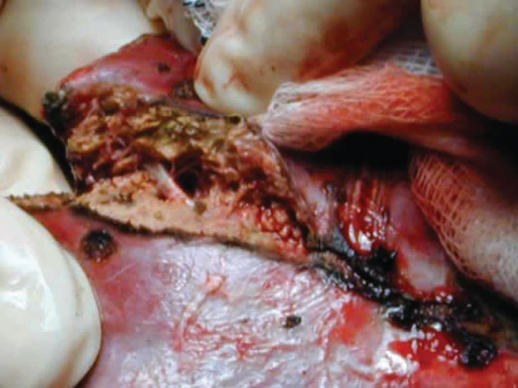
The In-Line radiofrequency ablation probe (Figure 2) is a 5 cm long plastic device and it comprises six metal electrodes spaced along the device, each 6 cm long. The electrodes can be deployed to any depth up to a maximum of 4 or 6 cm depending on the model used (Figures 3 and 4). The effective length of the coagulation is 5 cm and its width is 1 cm (Figure 5). The In-Line RFA device is bipolar and does not require a patient return plate.
Figure 2. .
Prototype In-Line RFA probe.
Figure 3. .
Prototype In-Line RFA deployment.
Figure 4. .
Prototype In-Line RFA deployment.
Figure 5. .
Avascular liver resection plane.
Before use, the resection line should be marked. In nonanatomic resection, we transect the liver along this line; in anatomic resection, after dividing the landmark vessels on the cutting surface, we deploy the In-Line RFA along this resection line. We strongly recommend that intraoperative ultrasound is used to ensure that there are no important structures in the chosen resection line. This is particularly important when a vascular biliary sheath crosses the resection plane and must be avoided. The In-Line RFA device will coagulate almost any structure that it encounters and this should not be deployed through or within 1 cm of a structure that must not be damaged.
During coagulation, the device will become very hot and the operator should hold the handle rather than the body of the device. A start out coagulation time of 3 min is used, after which the electrodes are twisted half a turn and pulled back individually. The probe is removed. The electrodes should be redeployed and cleaned if further coagulation is required. The device is re-used until the resection line has been completely treated – typically three applications are required for a right hepatectomy.
The parenchymal dissection of the coagulated transaction line is now completed using either the Kelly crush or a more sophisticated means of transection, such as the ultrasonic dissector or WaterJet. Large vessels and sheaths which are revealed by this dissection may then be ligated, sewn, clipped, or otherwise secured.
In our earlier experiments, we demonstrated that In-Line RFA-assisted liver resection could reduce blood loss dramatically 35,41. In a pilot study of eight patients where half of each resection was done with In-Line RFA and the remainder with an ultrasonic aspirator alone, we also reported a significant difference in blood loss (p=0.004) 44. From March 2002 to November 2004, a total of 38 patients underwent In-Line RFA-assisted liver resection, 12 were anatomic resections, the majority of the remaining resections were bisegmentectomy (Table II). The mean blood loss was 50±12.64 ml, and all of 38 patients had an uneventful recovery. During the postoperative period, no significant increase in bile leaks, abscess, or secondary hemorrhage was found 45; however, we accept that this is undoubtedly an issue that requires further monitoring.
Table II. Clinical details of In-Line RFA-assisted liver resection.
| No. | Sex | Date of treatment | Resection segments | Blood loss (ml) | Liver quality |
|---|---|---|---|---|---|
| 1 | F | 3/12/2002 | Left hepatectomy | 60 | Normal |
| 2 | M | 8/24/2002 | Right hepatectomy | 80 | Mild steatosis |
| 3 | M | 8/31/2002 | S 6,7 | 50 | Normal |
| 4 | M | 8/1/2003 | S 6,7 | 50 | Severe steatosis |
| 5 | M | 8/2/2003 | S 6 | 50 | Normal |
| 6 | F | 8/2/2003 | S 6,7 | 0 | Normal |
| 7 | F | 8/6/2003 | S 5,6 | 50 | Normal |
| 8 | F | 8/23/2003 | S 3 | 20 | Normal |
| 9 | M | 10/9/2003 | S 8 | 0 | Normal |
| 10 | M | 10/12/2003 | S 6,7 | 50 | Cirrhotic |
| 11 | F | 10/12/2003 | Right hepatectomy | 80 | Normal |
| 12 | F | 10/17/2003 | S 2,3 | 0 | Normal |
| 13 | F | 10/17/2003 | S 4a | 0 | Normal |
| 14 | F | 12/4/2003 | S 7,8 | 60 | Mild steatosis |
| 15 | M | 12/6/2003 | S 4,5,8 | 70 | Mild steatosis |
| 16 | F | 12/10/2003 | S 5,6 | 40 | Moderate steatosis |
| 17 | M | 12/11/2003 | S 4b | 0 | Mild steatosis |
| 18 | M | 12/12/2003 | S 4 | 0 | Mild steatosis |
| 19 | F | 12/20/2003 | Right hepatectomy | 90 | Normal |
| 20 | F | 12/21/2003 | S 5,6,7 | 50 | Moderate steatosis |
| 21 | M | 5/11/2004 | S 4b, 5,6 | 50 | Normal |
| 22 | F | 5/12/2004 | S 6 | 0 | Cirrhotic |
| 23 | F | 5/15/2004 | Extended left hepatectomy | 100 | Mild steatosis |
| 24 | M | 5/15/2004 | S 2,3 | 0 | Mild steatosis |
| 25 | M | 6/19/2004 | Left hepatectomy | 80 | Normal |
| 26 | M | 6/25/2004 | S 5,6,7 | 60 | Normal |
| 27 | F | 6/26/2004 | S 7-subs | 40 | Normal |
| 28 | F | 7/9/2004 | Right hepatectomy | 100 | Mild steatosis |
| 29 | M | 7/24/2004 | Left hepatectomy | 80 | Cirrhotic |
| 30 | M | 7/28/2004 | Right hepatectomy | 100 | Mild steatosis |
| 31 | M | 8/31/2004 | Right hepatectomy | 90 | Mild steatosis |
| 32 | M | 9/10/2004 | S 6,7 | 60 | Normal |
| 33 | F | 10/6/2004 | S 2, 3 | 0 | Mild steatosis |
| 34 | M | 10/7/2004 | S 2,3 | 0 | Normal |
| 35 | M | 10/14/2004 | S 7,6 | 50 | Mild steatosis |
| 36 | M | 10/21/2004 | Extended left hepatectomy | 150 | Mild steatosis |
| 37 | F | 11/2/2004 | Right hepatectomy | 100 | Normal |
| 38 | F | 11/7/2004 | S 2,3 | 40 | Normal |
Conclusion
RFA has been used to treat liver tumors for many years. In 1990 McGahan et al. and Rossi et al. used RFA to destroy hepatic tumors in animal livers 46,47. Since then, RFA has been used widely in the treatment of unresectable liver tumors 48,49,50,51. Furthermore, RFA of liver tumors is currently being performed percutaneously and laparoscopically 52,53,54,55.
The first description of RFA-assisted liver resection was published by Habib's group 38. This technique was clearly a major advance, and no morbidity and mortality were observed. It was shown that anesthetic time, operative time, hospital stay, and amount of blood loss could be decreased. Liver resection became a comparatively safer procedure. Subsequent studies demonstrated the merit of this technique 34,36,37,39,40,42,43.
Our In-Line RFA device is bipolar which has many advantages 56,57. The monopolar system is very slow approach, requiring many ablations to cover the designed resection plane. On the contrary, our In-Line device allows much more rapid (3 min for 6×5×1 cm) and precise treatment. The resection plane then can be dissected almost bloodlessly with an ultrasonic aspirator and only a few larger vessels and biliary ducts need to be ligated. Secondly, the bipolar device does not require grounding pads, because both electrodes are located on the probe and the alternating current circuit is confined within the target tissue.
Before use, the resection line should be marked. We recommend that intraoperative ultrasound is used to ensure that there are no important structures in the chosen resection line. This is particularly important when a vascular biliary sheath crosses the resection plane and must be avoided. The In-Line RFA device will coagulate almost any structure that it encounters and this should not be deployed through or within 1 cm of a structure that must not be damaged.
The In-Line device can similarly be used in segmental liver resection and nonanatomical resections. It is easy to learn to use the device. We believe the ability to sculpt a resection will be a useful tool in parenchymal sparing hepatic surgery, particularly in cirrhotics. The technique of RFA-assisted liver resection has a promising future.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–94. Lancet. 350;1997:1142–3. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 4.Farmer DG, Rosove MH, Shaked A, Busuttil RW. Current treatment modalities for hepatocellular carcinoma. Ann Surg. 1994;219:236–47. doi: 10.1097/00000658-199403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akriviadis EA, Llovet JM, Efremidis SC, Shouval D, Canelo R, Ringe B, et al. Hepatocellular carcinima. Br J Surg. 1998;85:1319–31. doi: 10.1046/j.1365-2168.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 6.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–9. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood TF, Rose DM, Chung M, Allegra DP, Fosbag J, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 8.Bilchik AJ, Wood TF, Allegra DP. Radiofrequency ablation of unresectable hepatic malignancies: lessons learned. Oncologist. 2001;6:24–33. doi: 10.1634/theoncologist.6-1-24. [DOI] [PubMed] [Google Scholar]
- 9.Harmon K, Ryan J, Biehl T, Lee F. Benefits and safety of hepatic resection for colorectal metastases. Am J Surg. 1999;177:402–4. doi: 10.1016/s0002-9610(99)00070-7. [DOI] [PubMed] [Google Scholar]
- 10.Ekberg H, Tranberg KG, Andersson R, Jeppsson B, Bengmark S. Major liver resection: perioperative course and management. Surgery. 1986;100:1–8. [PubMed] [Google Scholar]
- 11.Nagorney DM, Van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740–9. [PubMed] [Google Scholar]
- 12.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokudo N, Vera DR, Tada K, Koizumi M, Seki M, Matsubara T, et al. Predictors of successful hepatic resection: prognostic usefulness of hepatic asialoglycoprotein receptor analysis. World J Surg. 2002;26:1342–7. doi: 10.1007/s00268-002-6262-3. [DOI] [PubMed] [Google Scholar]
- 14.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–9. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 15.Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–22. doi: 10.1046/j.1365-2168.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanazaki K, Kajikawa S, Shimozawa N, Mihara M, Shimada K, Hiraguri M, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381–8. doi: 10.1016/s1072-7515(00)00700-6. [DOI] [PubMed] [Google Scholar]
- 17.Jensen LS, Andersen AJ, Christiansen PM, Hokland P, Juhl CO, Madsen G, et al. Postoperative infection and natural-killer-cell function following blood-transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513–16. doi: 10.1002/bjs.1800790613. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–87. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuzzo G, Giuliante F, Giovannini I, Tebala GD, de Cosmo G. Hepatic resections in normothermic ischemia. Surgery. 1996;120:852–8. doi: 10.1016/s0039-6060(96)80094-8. [DOI] [PubMed] [Google Scholar]
- 20.Tranberg KG, Rigotti P, Brackett KA, Bjornson HS, Fischer JE, Joffe SN. Liver resection. A comparison using Nd-YAG laser, an ultrasonic surgical aspirator, or blunt dissection. Am J Surg. 1986;151:368–73. doi: 10.1016/0002-9610(86)90471-x. [DOI] [PubMed] [Google Scholar]
- 21.Hansen PD, Isla AM, Habib NA. Liver resection using total vascular exclusion, scalpel division of the parenchyma and a simple compression technique for haemostasis and biliary control. J Gastrointest Surg. 1999;3:537–42. doi: 10.1016/s1091-255x(99)80109-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Ikai I, Kume M, Sakai Y, Yamauchi A, Shinohara H, et al. New technique for hepatic parenchymal resection using a Cavitron ultrasonic surgical aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23:1032–7. doi: 10.1007/s002689900619. [DOI] [PubMed] [Google Scholar]
- 23.Poon RT. Recent advances in techniques of liver resection. Surg Technol Int. 2004;13:71–7. [PubMed] [Google Scholar]
- 24.Chang YC. Low mortality major hepatectomy. Hepatogastroenterology. 2004;51:1766–70. [PubMed] [Google Scholar]
- 25.Mackenzie S, Dixon E, Bathe O, Sutherland F. Intermittent hepatic vein-total vascular exclusion during liver resection: anatomic and clinical studies. J Gastrointest Surg. 2005;9:658–66. doi: 10.1016/j.gassur.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Penchev RD, Kjossev KT, Losanoff JE. Application of a new water jet apparatus in open hepatobiliary surgery: hepatic resection, cholecystectomy, common bile duct lavage. Int Surg. 1997;82:182–6. [PubMed] [Google Scholar]
- 27.Wang WX, Fan ST. Use of the Endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003;26:193–6. doi: 10.1016/S1015-9584(09)60301-8. [DOI] [PubMed] [Google Scholar]
- 28.Hamazoe R, Maeta M, Murakami A, Yamashiro H, Kaibara N. Heating efficiency of radiofrequency capacitive hyperthermia for treatment of deep-seated tumors in the peritoneal cavity. J Surg Oncol. 1991;48:176–9. doi: 10.1002/jso.2930480307. [DOI] [PubMed] [Google Scholar]
- 29.Nicoli N, Casaril A, Mangiante G, Ciola M, Hilal MA, Marchiori L. Surgical treatment for liver metastases from colorectal carcinoma: results of 228 patients. Hepatogastroenterology. 2004;51:1810–14. [PubMed] [Google Scholar]
- 30.Llovet JM. Treatment of hepatocellular carcinoma. Curr Treat Options Gastroenterol. 2004;7:431–41. doi: 10.1007/s11938-004-0002-8. [DOI] [PubMed] [Google Scholar]
- 31.Head HW, Dodd GD., 3rd Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S167–S178. doi: 10.1053/j.gastro.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Gananadha S, Wulf S, Morris DL. Safety and efficacy of radiofrequency ablation of brain: a potentially minimally invasive treatment for brain tumours. Minim Invasive Neurosurg. 2004;47:325–8. doi: 10.1055/s-2004-830124. [DOI] [PubMed] [Google Scholar]
- 33.Steinke K, Sewell PE, Dupuy D, Lencioni R, Helmberger T, Kee ST, et al. Pulmonary radiofrequency ablation – an international study survey. Anticancer Res. 2004;24:339–43. [PubMed] [Google Scholar]
- 34.Navarra G, Lorenzini C, Curro G, Sampiero G, Habib NH. Radiofrequency-assisted hepatic resection – first experience. Ann Ital Chir 2004;75:53–6; discussion 56–7. [PubMed] [Google Scholar]
- 35.Gananadha S, Morris DL. Novel In-Line multielectrode radiofrequency ablation considerably reduces blood loss during liver resection in an animal model. Aust N Z J Surg. 2004;74:482–5. doi: 10.1111/j.1445-1433.2004.03035.x. [DOI] [PubMed] [Google Scholar]
- 36.Tepel J, Klomp HJ, Habib N, Fandrich F, Kremer B. Modification of the liver resection technique with radiofrequency coagulation. Chirurg. 2004;75:66–9. doi: 10.1007/s00104-003-0749-9. [DOI] [PubMed] [Google Scholar]
- 37.Navarra G, Lorenzini C, Curro G, Basaglia E, Habib NH. Early results after radiofrequency-assisted liver resection. Tumori. 2004;90:32–5. doi: 10.1177/030089160409000108. [DOI] [PubMed] [Google Scholar]
- 38.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beppu T, Ishiko T, Sugiyama S, Doi K, Yoshimura Y, Tanaka H, et al. Radio-frequency ablation (RFA) assisted endoscopic hepatectomy for hepatocellular carcinoma. Gan To Kagaku Ryoho. 2004;31:1740–2. [PubMed] [Google Scholar]
- 40.Weber JC, Navarra G, Habib NA, Bachellier P, Jaeck D. Laparoscopic radiofrequency-assisted liver resection. Surg Endosc. 2003;17:834. doi: 10.1007/s00464-002-4263-9. [DOI] [PubMed] [Google Scholar]
- 41.Haghighi KS, Steinke K, Hazratwala K, Kam PC, Daniel S, Morris DL. Controlled study of inline radiofrequency ablation (ILRFA) assisted transection of ovine liver. J Surg Res. 2005;123:139–43. doi: 10.1016/j.jss.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Stella M, Percivale A, Pasqualini M, Profeti A, Gandolfo N, Serafini G, et al. Radiofrequency-assisted liver resection. J Gastrointest Surg. 2003;7:797–801. doi: 10.1016/s1091-255x(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 43.Navarra G, Dpalding D, Zacharoulis D, Nicholls JP, Kirby S, Costa I, et al. Bloodless hepatectomy technique. HIPB. 2002;4:95–7. doi: 10.1080/136518202760378470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haghighi KS, Wang F, King J, Daniel S, Morris DL. In-Line radiofrequency ablation to minimize blood loss in hepatic parenchymal transection. Am J Surg. 2005;190:43–7. doi: 10.1016/j.amjsurg.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Yan TD, Kusyk T, Morris DL. A prospective study of In-Line Radiofrequency transection – its efficacy and complications. HPB 2006(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–70. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990;76:54–7. doi: 10.1177/030089169007600114. [DOI] [PubMed] [Google Scholar]
- 48.Matsuno N, Nakamura Y, Iwamoto H, Hama K, Akashi I, Konno S, et al. Radiofrequency ablation of unresectable hepatic malignancies. Chirurgia (Bucur) 2004;99:205–10. [PubMed] [Google Scholar]
- 49.Ng KK, Lam CM, Poon RT, Ai V, Tso WK, Fan ST. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol. 2003;18:616–29. doi: 10.1046/j.1440-1746.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 50.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–47. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 51.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg 2002;137:422–6; discussion 427. [DOI] [PubMed] [Google Scholar]
- 52.Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001;5:477–89. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- 53.Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127(5 Suppl 1):S159–S166. doi: 10.1053/j.gastro.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 54.Berber E, Senagore A, Remzi F, Rogers S, Herceg N, Casto K, et al. Laparoscopic radiofrequency ablation of liver tumors combined with colorectal procedures. Surg Laparosc Endosc Percutan Tech. 2004;14:186–90. doi: 10.1097/01.sle.0000136678.39377.86. [DOI] [PubMed] [Google Scholar]
- 55.White TJ, Roy-Choudhury SH, Breen DJ, Cast J, Maraveyas A, Smyth EF, et al. Percutaneous radiofrequency ablation of colorectal hepatic metastases – initial experience. An adjunct technique to systemic chemotherapy for those with inoperable colorectal hepatic metastases. Dig Surg. 2004;21:314–20. doi: 10.1159/000080886. [DOI] [PubMed] [Google Scholar]
- 56.Tacke J, Mahnken A, Roggan A, Gunther RW. Multipolar radiofrequency ablation: first clinical results. Rofo. 2004;176:324–9. doi: 10.1055/s-2004-812723. [DOI] [PubMed] [Google Scholar]
- 57.Hamner CE, Lutterman A, Potter DD, Sundt TM, III, Schaff HV, Francischelli D. Irrigated bipolar radiofrequency ablation with transmurality feedback for the surgical Cox-Maze procedure. Heart Surgery Forum. 2003;6:418–23. [PubMed] [Google Scholar]