Abstract
Background. Bleeding during liver transection remains a potential hazard. This study aims to report the efficacy and complications of in-line radiofrequency ablation (ILRFA)-assisted liver resection. Patients and methods. The blood loss of 25 consecutive patients who underwent ILRFA-assisted liver resection was obtained by weighing swabs and measuring suction jar contents during liver resection and calculated in ml per cm2 of the transection surface area. Postoperative complications were recorded. Five clinical variables, which might affect blood loss, were analyzed. Results. The mean blood loss during parenchymal dissection for the ILRFA group was 3.4±3.2 ml/cm2. Three patients had intra-abdominal collections, including one patient with bile leakage after ILRFA-assisted liver resection. Age, gender, extent of liver resection, liver quality and Pringle maneuver did not demonstrate significant impact on blood loss. Conclusions. This study showed that ILRFA-assisted liver resection was associated with very low blood loss. This is likely to improve the operative safety of liver resection for hepatic tumors. There were no significant postoperative sequelae.
Keywords: liver resection, blood loss, bile leak, radiofrequency ablation
Introduction
Blood loss during liver resection remains a potential problem. It has been shown to have a deleterious impact on both short- and long-term outcomes of the surgery 1,2. Median blood loss is reported as 450–1500 ml following liver resection, depending on the extent of resection and the normality of the liver parenchyma 3,4,5,6,7. Perioperative transfusion for major blood loss is also associated with adverse prognosis in patients who undergo liver resection 8.
Radiofrequency ablation (RFA) has now been widely used for in situ destruction of liver tumors. RF electrical current produces heat, coagulation, and local tissue necrosis. Some surgeons have described use of an original RFA needle to create an avascular area through which the division of the hepatic parenchyma can be achieved with minimal blood loss 9,10. The recently designed in-line radiofrequency ablation (ILRFA) probe consists of a plastic body with six deployable electrodes in one line, which can produce a precise avascular section up to 5 cm long, 6 cm deep and 1 cm wide. This transection plane can then be dissected almost bloodlessly with only a few large vessels and biliary structures requiring ligation or application of clips 11,12.
Some other techniques of hepatic resection, such as microwave coagulation and harmonic scalpel, have been shown to cause more bile leakage than the conventional clamp crushing method 9,13. It is unknown if the area of necrosis created with ILRFA would result in a higher incidence of postoperative bile leakage. With our previous pilot study design, it was not possible to report on the postoperative complications associated with ILRFA-assisted liver resection 12. In this article, we report the efficacy and complications of ILRFA-assisted liver resection in the next 25 consecutive patients with primary or metastatic liver tumors.
Patients and methods
The Ethics Committee of SESAHS approved this study (Approval No: 03/172).
Materials
The ILRFA probe is a 5 cm long plastic device with six electrodes, spaced along the probe in one line, each 6 cm long, which can be deployed to varying depths in the hepatic parenchyma (Figure 1). The probe is connected to a standard RFA generator (1500 RITA generator, Sunnyvale, CA, USA). Each ablation was performed strictly according to the ILRFA protocol and each ablation cycle was 3 min.
Figure 1. .
In-line radiofrequency ablation (ILRFA) probe.
Patient selection
The inclusion criteria for ILRFA-assisted liver resection were: (1) patients with radiologically or histologically proven primary or secondary hepatic tumors; (2) hepatic disease suitable for liver resection; (3) signed informed consent for ILRFA; (4) age between 18 and 85 years. The exclusion criteria were: (1) previous hepatic operations; (2) bleeding diathesis (prothrombin time > 1.5 and platelets < 100×109); (3) additional procedures planned to the liver, e.g. cryoablation. A total of 51 patients underwent liver surgery during the study period; 25 patients who satisfied both the inclusion and exclusion criteria underwent ILRFA-assisted liver resection.
Operative procedures
All operations were performed under general anesthesia without central venous pressure (CVP) reduction. After liver mobilization, intraoperative ultrasound (IOUS) was performed to confirm the size, number and location of the hepatic tumors in relation to the major hepatic veins and the portal sheaths. The plane of transection was marked with a diathermy on the surface of the liver.
The IOUS was also used to identify the relationship of the hepatic veins and the portal sheaths to the intended transection plane. Under IOUS guidance, these intraparenchymal structures were avoided during the deployment of the ILRFA electrodes, as each electrode can be independently deployed to varying depths. All the intended transection planes were ablated with ILRFA and then transected using an ultrasonic aspirator (Selector, Integra Neurosciences, UK). Blood loss during transection was calculated in ml/cm2, where the amount of blood loss was measured in milliliters by weighing the sponges and counting the volume in the suction system, and the transection area was measured on the specimen in cm2. In some of the patients, the Pringle maneuver was applied during the liver resection. The larger vessels and bile ducts were cauterized, clipped or ligated according to our routine practice.
All procedures were performed by D.L.M., an experienced liver surgeon. The clinical information in this study was collected prospectively by completing a standardized data sheet for each patient. Patients were followed at monthly intervals for the first 6 months and then at 3-monthly intervals thereafter. The assessments included clinical examination, liver function tests, tumor marker analysis and abdominal computed tomography (CT).
Statistical analysis
Statistical analysis was performed on the prospectively collected clinical data. The efficacy of ILRFA in blood loss was studied. The postoperative complications were recorded. Wilcoxon rank test was used for the comparison of difference in means of continuous factors. The statistical analyses were performed using SPSS for Windows (Version 11.5; SPSS GmbH, Munich, Germany). A significant difference was assumed for p values < 0.05.
Results
All operations proceeded as planned. The demographics of these patients are shown in Table I. There was no hospital mortality. The mean volume of blood loss during the liver transection was 273±325 ml. Twelve patients had less extensive liver resection (<3 segments); their absolute volume of blood loss during liver transection was 35, 50, 50, 50, 87, 150, 205, 245, 295, 300, 305 and 445 ml, respectively; and their mean volume of blood loss during liver transection was 185±135 ml. The remaining 13 patients had more extensive liver transection (≥ 3 segments); their absolute volume of blood loss during liver transection was 50, 60, 80, 95, 100, 105, 200, 210, 350, 350, 605, 895 and 1500 ml, respectively; and their mean volume of blood loss was 354±425 ml (p=0.300, vs <3 segments). The mean volume of blood loss per cm2 was 3.4±3.2. Seven patients (28%) had at least one unit of intraoperative blood transfusion and the average unit of transfusion given was 0.6 units per patient. There were 15 patients (60%) with normal liver, 8 patients (32%) with steatotic liver and 2 patients (8%) with cirrhotic livers. The blood loss of the two cirrhotic patients was 2.5 and 3.3 ml/cm2, respectively. The blood loss of the eight steatotic patients was 1, 1.2, 1.2, 1.3, 1.7, 2.0, 5.0, and 15.0 ml/cm2, respectively. The mean operative time was 169±58 min. The mean postoperative stay was 7±4 days. The median follow-up was 4 months (range 1–11 months).
Table I. Patient characteristics (n=25).
| Clinical factor | No. of patients | % |
|---|---|---|
| Overall | 25 | 100 |
| Gender | – | – |
| Male | 16 | 64 |
| Female | 9 | 36 |
| Age (years) | – | – |
| <60 | 10 | 40 |
| ≥60 | 15 | 60 |
| Liver quality | – | – |
| Normal | 15 | 60 |
| Steatotic | 8 | 32 |
| Cirrhotic | 2 | 8 |
| Resection type* | – | – |
| Right hepatectomy | 8 | 32 |
| Left hepatectomy | 2 | 8 |
| Extended left hepatectomy | 1 | 4 |
| Left lobectomy | 5 | 20 |
| Segmental resection | 9 | 36 |
| In-flow occlusion | – | – |
| No | 14 | 56 |
| Yes | 11 | 44 |
| Blood transfusion | – | – |
| No | 18 | 72 |
| Yes | 7 | 28 |
*Terminology of Couinaud, 1957.
The impact of five variables that might potentially affect blood loss during ILRFA-assisted liver transection is demonstrated in Table II. They are gender, age (<60 vs ≥ 60 years), liver quality (normal vs steatotic and cirrhotic), extent of resection (<3 segments vs ≥ 3 segments), and Pringle maneuver application. None of the five factors demonstrated any significant impact on blood loss during ILRFA-assisted liver transection. Eleven patients (44%) had the Pringle maneuver applied during the liver resection, but it was used only selectively in difficult cases. The average duration of clamping was 19 min. This maneuver failed to show statistically significant impact on blood loss (p=0.632).
Table II. Five factors that potentially affect blood loss during ILRFA-assisted liver transection.
| Clinical factor | No. | Blood loss (ml/cm2) Mean±SD | p value* |
|---|---|---|---|
| Overall | 25 | 3.4± 3.2 | – |
| Gender | – | – | 0.379 |
| Male | 16 | 3.9±3.7 | – |
| Female | 9 | 2.5±2.0 | – |
| Age (years) | – | – | 0.739 |
| < 60 | 10 | 4.0±4.4 | – |
| ≥ 60 | 15 | 3.0±2.2 | – |
| Liver quality | – | – | 0.523 |
| Normal | 15 | 3.4±2.5 | – |
| Steatotic/cirrhotic | 10 | 3.4±4.3 | – |
| Extent of resection | – | – | 0.531 |
| < 3 segments | 12 | 3.4±2.7 | – |
| ≥ 3 segments | 13 | 3.4±3.7 | – |
| In-flow occlusion | – | – | 0.632 |
| No | 14 | 3.7±2.5 | – |
| Yes | 11 | 3.0±4.0 | – |
*Wilcoxon rank test for the comparison of difference in means of blood loss.
Three patients had postoperative intra-abdominal collections; including one patient who had a bile collection. All these patients were managed with CT-guided drain insertion. The median interval between the operation and the complication was 5 days (range 2–20 days). There were no other postoperative complications detected at the last follow-up.
Discussion
Studies show that 20–60% of patients undergoing liver resection require blood transfusion 6,14. In patients with cirrhotic livers, bleeding is a more significant issue. Both short- and long-term morbidity and mortality correlate with intraoperative blood loss 1,2. The more recent study from the Memorial Sloan-Kettering group reported an estimated blood loss of 871±24 ml, a transfusion rate of 49%, a mean in-flow occlusion time of 31 min, and a mean transfusion of four units. Blood loss in patients who died was nearly three times greater 4.
Over the years, different techniques have been described to minimize blood loss during the transection phase. Surgeons use low CVP anesthesia, continuous or intermittent Pringle maneuver or total vascular exclusion in trying to achieve better operative results. Parenchymal transection can be performed with finger fracture, a scalpel or the more refined ultrasonic aspirator, which disaggregates and sucks away the soft hepatic parenchymal tissues, leaving the larger and tougher vessels and ductal structures behind for ligation or clipping. Rau et al. reported a blood loss of 24.3 ml/cm2 of resection surface using the ultrasonic aspirator 15. In cirrhotic livers, due to dense fibrosis, the ultrasonic aspirators are less effective in dividing the parenchymal tissue without bleeding 15. Water-jet cutters (hydrodissectors) have also been used and 17.7 ml/cm2 of blood loss has been reported 15. However, early postoperative bleeding and bile leakage still exist, probably because of inadequate ligation of small vessels or bile ducts after segregation of the plane with these devices.
Habib and co-workers first described the use of an unmodified RFA probe to create a 2 cm zone of necrosis by repeatedly ablating tissue in the transection plane, followed by parenchymal dissection with a surgical scalpel 9. They showed very encouraging results. The mean blood loss was 30±10 ml and no morbidity or mortality was observed 9. However, the major concern with this approach is that it is relatively slow, requiring many ablations to cover the intended transection plane, and the spherical ablations are not ideal for developing a coagulative transection plane. Multiple independent ablations have to be used, and this can be time-consuming. In addition, to achieve an adequate transection plane with this unmodified RFA probe, surgeons sometimes have to sacrifice more normal liver tissue, which can be problematic in cirrhotics and in complex liver resections 10.
This study reports the preliminary results of an innovative procedure using ILRFA to assist liver resection. In a sheep model, we have demonstrated that ILRFA could produce an avascular plane up to 6 cm deep and 5 cm long 11. Dissection or cutting through this area was then achieved with minimal, if any, blood loss. We have also demonstrated a significant difference in blood loss (p=0.004) in a pilot study of eight patients, who had ILRFA for half of the transection plane and the other half was transected using a conventional ultrasonic aspirator alone 12.
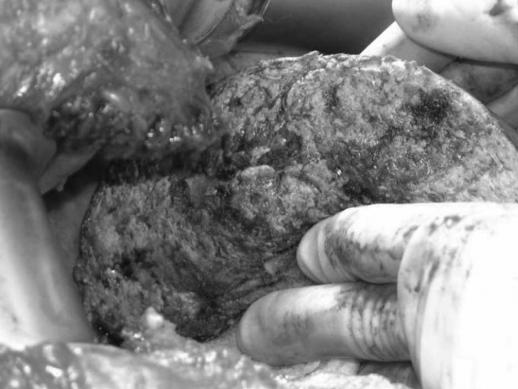
The major advantages of ILRFA are that it is very precise and rapid. Each ILRFA electrode should be deployed individually, under the guidance of intraoperative ultrasound, to avoid penetrating any major vessels (Figure 2). The RF field created between electrodes of alternating polarity results in localized heating and coagulation of tissue including blood vessels up to approximately 3 mm. The ILRFA probe can be withdrawn and re-applied along the intended transection plane (Figure 3). Each RF ablation requires only 3 min to achieve a complete coagulative ablation. This ablated plane can then be dissected almost bloodlessly with an ultrasonic aspirator (Figure 4). The small blood vessels and biliary structures are coagulated in the process and the larger ones are ligated or clipped.
Figure 2. .
In-line radiofrequency ablation (ILRFA) probe deployment prior to ablation.
Figure 3. .
In-line radiofrequency ablation (ILRFA) creating a coagulated section up to 5 cm long, 6 cm deep and 1 cm wide prior to liver resection.
Figure 4. .
Avascular transection surface after in-line radiofrequency ablation (ILRFA)-assisted liver resection.
In this study, the mean blood loss was 3.4±3.2 ml/cm2. The results are lower than those in our previous pilot study, perhaps due to our increased experience 12. The blood loss of the two cirrhotic patients was 2.5 and 3.3 ml/cm2, respectively, and there were another two cirrhotics in our pilot study 12. It is difficult to draw conclusions from such a small number of patients, but it is most encouraging that ILRFA appears to work very effectively in this difficult group of patients.
Some other techniques of hepatic resection, such as microwave coagulation and harmonic scalpel, have been shown to cause more bile leakage than the conventional clamp crushing method 9,13. One of the concerns, however, which our previous study was unable to address, was the incidence of bile leakage after ILRFA 12. In the present study, one patient had biliary leakage and there was no other significant postoperative sequela.
This procedure is easy to learn and surgeons with a good knowledge of liver anatomy can apply it to both segmental and formal resections. We have used this technique in both major and minor liver resections. The extent of liver transection did not affect blood loss in this study (p=0.531). However, its most important limitation is that the radiofrequency energy will ablate any tissue that it is deployed through. Therefore it is of great importance not to damage hepatic veins or portal sheaths to the liver, which are to be retained. To avoid this, we recommend the use of intraoperative ultrasound during the electrode deployment.
References
- 1.Nagorney DM, Van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancies. Surgical management and determinants of survival. Surgery. 1989;106:740–9. [PubMed] [Google Scholar]
- 2.Makuuchi M, Takayama T, Gunvèn P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644–8. doi: 10.1007/BF01658893. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–87. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection – analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin RCG, II, Jarnagin WR, Fong Y, Biernacki P, Blumgart LH, DeMatteo RP. The use of fresh frozen plasma after major hepatic resection for colorectal metastasis: is there a standard for transfusion? J Am Coll Surg. 2003;196:402–9. doi: 10.1016/S1072-7515(02)01752-0. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–6. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 7.Melendez J, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, et al. Perioperative outcomes of major hepatic resection under low central venous pressure anesthesia: blood loss, blood transfusion and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 8.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:869–70. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stella M, Percivale A, Pasqualini M, Profeti A, Gandolfo N, Serafini G, et al. Radiofrequency-assisted liver resection. J Gastrointest Surg. 2003;7:797–801. doi: 10.1016/s1091-255x(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 11.Gananadha S, Morris DL. Novel in-line multi-electrode radiofrequency ablation considerably reduces blood loss during liver resection in an animal model. Aust N Z J Surg. 2004;74:482–5. doi: 10.1111/j.1445-1433.2004.03035.x. [DOI] [PubMed] [Google Scholar]
- 12.Haghighi KS, Wang F, King J, Daniel S, Morris DL. In-line radiofrequency ablation to minimize blood loss in hepatic parenchymal transection. Am J Surg. 2005;190:43–7. doi: 10.1016/j.amjsurg.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Ahmad SA, Lowy AM, Buell JF, Pennington LJ, Soldano DA, et al. Increased biliary fistulas after liver resection with the harmonic scalpel. Am Surg. 2003;69:815–19. [PubMed] [Google Scholar]
- 14.Arnoletti JP, Brodsky J. Reduction of transfusion requirements during major hepatic resection for metastatic disease. Surgery. 1999;125:166–71. [PubMed] [Google Scholar]
- 15.Rau HG, Schardey HM, Buttler E, Reuter C, Cohnert TU, Schildberg FW. A comparison of different techniques for liver resection: blunt dissection, ultrasonic aspirator and jet-cutter. Eur J Surg Oncol. 1995;21:183–7. doi: 10.1016/s0748-7983(95)90435-2. [DOI] [PubMed] [Google Scholar]