Abstract
Background. The aim of this study was to evaluate the postoperative morbidity and, in the medium-term results, the incidence of relapses in the laparoscopic treatment of non-parasitic hepatic cysts (NPHC) and polycystic liver disease (PCLD). Patients and methods. From 1999 to 2003, 12 patients with NPHC and 3 patients with PCLD with few large cysts in the anterior hepatic segments underwent laparoscopic fenestration and deroofing. Results. There were no conversions and no mortality; the mean operative time was 55 min for NPHC and 120 min for PCLD. Postoperative morbidity comprised two patients with bronchopneumonic infiltrations and in one patient with PCLD ascites resolved spontaneously. All the patients experienced resolution of the symptomatology. Follow-up was carried out from 3 to 38 months (mean 18 months). There was no relapse of the disease. Discussion. The preoperative selection of patients is fundamental to program the surgical treatment. Laparoscopy can be considered a safe and efficacious treatment for NPHC and PCLD.
Keywords: Non-parasitic hepatic cyst, polycystic liver disease, laparoscopic resection, laparoscopic fenestration, laparoscopic deroofing
Introduction
The treatment of simple hepatic cysts presents some relevant controversies. There is no agreement about the possibility of identifying, in the preoperative phase, simple cysts and polycystic hepatic disease as distinct from the cases with degeneration (cystoadenoma, cystoadenocarcinoma). The surgical indication and the choice of the type of surgical treatment, with possible laparoscopic approach, on the basis of the immediate results (postoperative morbidity) and of the medium-term results (possibility of relapse of the disease), are also under discussion. The classification of hepatic cysts includes congenital and acquired forms. We distinguish among the congenital, the simple or sporadic forms, and polycystic disease; the congenital forms can be associated with renal cysts. The acquired forms include cysts as an evolution of organized traumatic hematomas, parasitic cysts, neoplastic cysts (cystoadenoma, cystoadenocarcinoma), and metastatic cysts. The aim of this study was to evaluate, in a small but homogeneous series (from a single institution), the postoperative morbidity and, in the medium-term results, the incidence of relapses in the laparoscopic treatment of nonparasitic hepatic cysts (NPHC) and of polycystic liver disease (PCLD).
Patients and methods
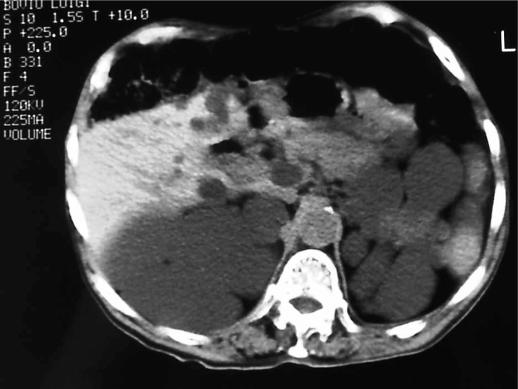
Fifteen patients were examined and treated in our institution during the period 1999–2003. There were 12 patients with simple hepatic cysts and only 3 with PCLD (Figure 1); 6 males and 9 females, mean age 54 years (range 34–61 years). Tables I and II show the patients’ symptoms and Table I shows the sizes of simple cysts (mean 12 cm, range 9–16 cm).
Figure 1. .
Table I. Clinical findings in patients with NPHC.
| Patient no. | Cyst/s size (cm) | Symptoms |
|---|---|---|
| 1 | 13 | Epigastric pain, gallbladder lithiasis |
| 2 | 10/4 | Epigastric pain, gallbladder lithiasis |
| 3 | 9/7 | Abdominal pain |
| 4 | 12 | Abdominal and right shoulder pain |
| 5 | 14 | Abdominal pain |
| 6 | 10/3 | Right shoulder pain |
| 7 | 9 | Epigastric pain |
| 8 | 16 | Right shoulder pain + gallbladder lithiasis |
| 9 | 6 | Epigastric pain |
| 10 | 8 | Abdominal pain |
| 11 | 11/4 | Abdominal and right shoulder pain |
| 12 | 20/4 | Abdominal pain + gallbladder lithiasis |
Table II. Clinical findings in patients with PCLD type 1.
| Patient no. | Symptoms |
|---|---|
| 13 | Abdominal pain, dyspnea |
| 14 | Abdominal pain + gallbladder lithiasis |
| 15 | Abdominal pain |
In all patients the clinical scenario was upper abdominal quadrant pain, with irradiation to the right shoulder in some cases. The smart type of pain, referred to the presence of the cysts, was persistent and severe, lasting for several months. All the patients visited their primary care physician and underwent an ultrasonographic evaluation before admission to the hospital. As a result, the hepatic cysts were evident and in five cases the addition of cholelithiasis was noted. On admission to our clinical institute, morphologic definition of the cystic lesions was determined by CT and/or MRI examination, in order to exclude parasitic or neoplastic origin and define the topography of the lesions in the liver. The diagnosis was completed by investigating tumor markers and echinococcus serology. There was evidence of three cases of polycystosis type 1. The indication for surgical treatment, then, was based on the presence of an evident symptomatology. All the patients were submitted to a laparoscopic approach: the wide fenestration technique (Lin procedure) 1 by deroofing the cyst wall was the preferred procedure. Cholecystectomy was added in case of lithiasis. The laparoscopic fenestration technique was also applied to the patients with PCLD type 1.
The treated lesions mainly involved anterior hepatic segments (II, III, IV, and V), with the cystic walls widely superficial on the liver, although in two cases the cysts were located posterior-laterally, but they were accessible by the laparoscopic approach. The deeply localized cysts and those bordering with the superficial ones were fenestrated a second time and through the superficial cysts, after an explorative puncture. The cutting of the cystic wall was performed by means of electronic and ultrasonic scalpel, and the surgeons avoided going too far into the hepatic parenchyma. The residual cavity was widely in communication with the peritoneum and then drained. We registered no cases of intraoperative biliary leakage in the treated cysts. In situ omentoplasty was added in the two posteriorly located cases.
Results
There were no conversions in the intraoperative phase for either pathology. There was no mortality. The mean operative time for the polycystic disease was longer (120 min) than for the simple cysts (55 min). This difference must only be attributed to the necessity of treating numerous cysts, and not to a more difficult or different intervention for the polycystosis. The postoperative stay, the complete resolution of the symptomatology and the histologic results, in all the cases of biliary cysts on the segments of the examined cystic wall, were the same for both pathologies. There were a few minor and nonspecific postoperative complications, such as pleural effusions or bronchopneumonic infiltrations. Postoperative ascites is a more important problem, as emphasized in the literature 2. In our series, this complication occurred in one of the patients treated for polycystic disease (Table III). Moreover, the medium-term results were the most interesting. In this series we evaluated the medium-term results with a mean follow-up of 18 months after the surgical intervention (range 3–38 months). There was a follow-up every 6 months (except for the last patients), and, as well as clinical observation, it included laboratory investigations and abdominal ultrasonographic examination. The results confirmed the absence of both the symptomatology and the relapse of the disease.
Table III. Immediate results with the laparoscopic approach.
| Parameter | NPHC | PCLD (type 1) |
|---|---|---|
| Conversion | No | No |
| Mortality | No | No |
| Mean operative time (min) | 55 (range 40–90) | 120 (range 80–150) |
| Bronchopneumonic | ||
| Infiltrations | 1 | 1 |
| Pleural effusion | 1 | 1 |
| Ascites | No | 1 |
| Hospital stay (days) | 6 (range 4–14) | 6 (range 4–14) |
| Resolution of the | ||
| symptomatology | Complete | Complete |
| Histology of the removed wall | Simple cyst | Simple cyst |
Discussion
Two elements of debate are in evidence: the anatomical-clinical characteristics of the pathology and the surgical indication. First of all, it is useful to define the general clinical characteristics of the pathology, to establish an appropriate and adequate therapeutic program. In fact, there are three fundamental points: this is a benign pathology, often asymptomatic or with few signs and symptoms (vague abdominal pain, mild dyspnea, etc.), and this is accompanied by normal hepatic function tests. Then, apart from the open or laparoscopic approach (as the interventions can readily be overlapped), the surgical indication in general must be established on the basis of the appearance of the symptoms. These are related to the presence of hepatic cysts, i.e. the presence of another disease such as cholelithiasis. The clinical scenario can be overlapping, both in simple cysts and in polycystosis. Generally the reported symptoms derive from the effect of the volume of the cyst(s). Moreover, about 15% 3 of patients are asymptomatic, but the most common problem is the sense of heaviness and the mild pain in the upper abdominal quadrants. Among the complications we noticed bleeding, perforation, torsion of pedunculated cysts: these are all unusual in the evolution of the disease. Although rare, infection of the cysts (more frequent in polycystosis) and the appearance of jaundice from compression of the biliary tree can be found 4. The number and, above all, the size of the cysts, such as simple cysts and polycystosis type 1, must be considered first. The therapeutic approach for polycystosis type 2 is different. As mentioned above, the symptomatology related to the cysts is vague and undefined. We registered, in particular, epigastric or right shoulder pain, precocious sense of gastric fullness, and clinostatic dyspnea; in most cases there was an indeterminate abdominal pain. There was the same clinical scenario in polycystosis, where the painful symptomatology and the abdominal distension, clinostatic dyspnea, and fatigue with limitation of physical activity because of the hepatomegaly, are in evidence 2.
The surgical indication must be related to the presence of the biliary cysts and not to other concomitant pathologies (gastro-esophageal reflux, cholelithiasis, etc.). In particular, in many cases the hepatic cysts or the polycystosis are asymptomatic. Subsequently, it is fundamental to define with certainty the nature of the simple hepatic cysts, by excluding a parasitic etiology and also the suspicion of malignancy. This is achieved by evaluation of the regularity of the cystic wall, densitometric characteristics of the content, and characteristics of the surrounding hepatic parenchyma. In addition to the preoperative study, serology for echinococcosis and investigation of tumor markers must be carried out. Finally it must be determined that the surgical indication is for cystic hepatic lesions that are responsible for the clinical scenario and that they are definitely nonparasitic and non-neoplastic. When the surgical indication is established, it is necessary to choose the type of treatment. Nonsurgical procedures were proposed for simple cysts; percutaneous aspiration, occasionally executed, has no therapeutic role because relapses reach the 100% level 5,6. Percutaneous aspiration, followed by the injection of 95% ethanol or other sclerosing substances, is also followed by a high incidence of relapses 5,6,7. Therefore, the value of this therapeutic procedure is very debatable, especially in the light of the disappointing results.
In this perspective, surgical treatment remains in evidence, and its modality was codified by Lin et al. 1 in the past. More recently the same treatment of fenestration and deroofing was proposed and largely carried out by the laparoscopic approach and it was largely recognized as a safe and feasible procedure. Open deroofing should be reserved for the treatment of inaccessible cysts by laparoscopy. On the whole, surgical therapy with fenestration by deroofing of the cystic wall (executed by both the open and laparoscopic approach) is recognized as the therapy of choice for the treatment of simple hepatic cysts because of the low morbidity rates, the high level of effectiveness and the very small relapse index 3,8,9,10,11. Hepatic resection is indicated as a second level choice, after a first deroofing but with relapse of disease and/or complications 12. In addition, hepatic resection is the preferred treatment for patients with multiple cysts or in PCLD and with a wide hepatic involvement 13, but leaving a sufficient amount of hepatic parenchyma. The hepatic resection almost invariably requires an open approach.
The therapeutic program for the polycystic liver is obviously more complex. Preliminarily, it is useful to consider the classification proposed by Morino et al. 2 that distinguishes, among the PCLD cases, a type 1 characterized by a limited number of large-sized cysts located in the anterior hepatic segments, and a type 2 characterized by a great number of cysts throughout the liver. A further subdivision among the PCLD cases was suggested by Gigot et al., which distinguishes type 1, with large cysts (>10 cm), in limited number (<10); type 2 with multiple and widespread cysts of moderate size, but with largely undamaged parenchyma; type 3 with widespread small cysts, but with small amounts of undamaged residual parenchyma 14. In conclusion, type 1 is almost the same as the simple hepatic cysts in the anatomical-clinical field, and then there are the same problems of surgical indication. For the latter, any possible suspicion regarding malignancy must be investigated by study of the morphology of the internal surfaces and the parenchyma surrounding the cysts. The programmable surgical intervention is the same for both simple cysts and polycystosis type 1, represented by fenestration and deroofing, which is also perfectly feasible by the laparoscopic approach 13. Moreover if there is a prevalence of the cysts in one sector, a resective procedure can be proposed.
The therapeutic program for polycystosis type 2 (Gigot's type 3) is much more complicated, so the perspective of transplantation is very real. In particular, patients with congenital hepatic fibrosis and with extensive substitution of the parenchyma by the cysts (polycystosis type 2), are candidates for transplantation. Briefly, hepatic transplantation is generally reserved for cases of polycystosis with severe hepatic failure and/or in case of relapse after previous hepatic resections. In addition, some of these patients also suffer from polycystic kidney and kidney failure, so combined kidney/liver transplantation can be the appropriate treatment 15,16,17,18. The reliability and safety of the intervention of fenestration and deroofing of the cysts are well established now, and this procedure can be executed with the same results by both laparoscopic and open approaches. The fenestration and deroofing is not very diffferent in the treatment of both the hepatic polycystosis and the simple cysts, so the relapse incidence is unimportant. Surgical indication requires a careful selection of the patients, recognizing the type 1 cases, overlapping with simple cysts, for which the laparoscopic fenestration and deroofing are also safe and efficacious.
In conclusion, there is a very low incidence of relapses after the treatment of simple cysts. Simple cysts are quite frequent in polycystosis because the development of new cysts replaces the fenestrated and decompressed cysts. The increase of the cyst volume is blocked by the increase of the endo-abdominal pressure and the surgical decompression of the cysts can restart the development of others 2,19. A particular consideration must be reserved for the possibility of the appearance of ascites in the postoperative phase that derives from the well fenestrated cystic cavities, and this ends with the peritoneal absorption. In this condition the minimally invasive approach, as in the laparotomy procedures, shows the advantage of small-sized access points that are easily suturable, thus avoidig leakage of ascitic liquid from the laparotomy wound 2,13.
The laparoscopic approach, with fenestration and deroofing, in simple hepatic cysts and in type 1 polycystosis, presents considerable advantages as regards the immediate and medium-term results, if we compare them to the same results obtained during our previous experience with laparotomy. This is largely confirmed by the litera ture: reduction of postoperative pain, more comfort, early mobilization, shorter hospital stay, considerable esthetic advantage, but above all, the resolution of the symptomatology and the low incidence of distant relapse of disease 20,21,22,23. Thus, preoperative selection of patients with simple hepatic cysts and with type 1 polycystosis is fundamental to program the surgical treatment. Laparoscopic fenestration and deroofing can also be considered a safe and efficacious procedure for the relapses, particularly for simple hepatic cysts, but also for polycystic disease with large and superficial cysts 20,21,22,23,24.
References
- 1.Lin TY, Chen CC, Wang SM. Treatment of non-parasitic cystic disease of the liver: a new approach to therapy with polycystic liver. Ann Surg. 1968;168:921–7. doi: 10.1097/00000658-196811000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morino M, De Giuli M, Festa V, Garrone C. Laparoscopic management of symptomatic nonparasitic cysts of the liver. Indications and results. Ann Surg. 1994;219:157–64. doi: 10.1097/00000658-199402000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gigot JF, Hubert C, Banice R, Kendrick ML. Laparoscopic management of benign liver disease: where are we? HPB. 2004;6:197–212. doi: 10.1080/13651820410023950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parks RW, Garden OJ. In:Hepatobiliary and pancreatic surgery. London: WB Saunders, 2001:75–105. [Google Scholar]
- 5.Kairaluoma MI, Leinonen A, Stahlberg M, Paivansalo M, Kiviniemi H, Siniluoto T. Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg. 1989;210:208–15. doi: 10.1097/00000658-198908000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montorsi M, Torzilli G, Fumagalli U, Bona S, Rostai R, De Simone M, et al. Percutaneous alcohol sclerotherapy of simple hepatic cysts. Results from a multicentre survey in Italy. HPB Surg. 1994;8:89–94. doi: 10.1155/1994/10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regev A, Reddy KR, Berho M, Sleeman D, Levi JU, Livingstone AS, et al. Large cystic lesions of the liver in adults: a 15-year experience in a tertiary center. J Am Coll Surg. 2001;193:36–45. doi: 10.1016/s1072-7515(01)00865-1. [DOI] [PubMed] [Google Scholar]
- 8.Moorthy K, Mihssin N, Houghton PW. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl. 2001;83:409–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Zacherl J, Imhof M, Fugger R, Fritsch A. Laparoscopic unroofing of symptomatic congenital liver cysts. Surg Endosc. 1996;10:813–15. doi: 10.1007/BF00189540. [DOI] [PubMed] [Google Scholar]
- 10.Kwon AH, Matsui Y, Inui H, Imamura A, Kamiyama Y. Laparoscopic treatment using an argon beam coagulator for nonparasitic liver cysts. Am J Surg. 2003;185:273–7. doi: 10.1016/s0002-9610(02)01361-2. [DOI] [PubMed] [Google Scholar]
- 11.Hansman MF, Ryan JA, Jr, Holmes JH, 4th, Hogan S, Lee FT, Kramer D, et al. Management and long-term follow-up of hepatic cysts. Am J Surg. 2001;181:404–10. doi: 10.1016/s0002-9610(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 12.Tocchi A, Mazzoni G, Costa G, Cassini D, Bettelli E, Agostini N, et al. Symptomatic nonparasitic hepatic cysts: options for and results of surgical management. Arch Surg. 2002;137:154–8. doi: 10.1001/archsurg.137.2.154. [DOI] [PubMed] [Google Scholar]
- 13.Martin IJ, McKinley AJ, Currie EJ, Holmes P, Garden OJ. Tailoring the management of nonparasitic liver cysts. Ann Surg. 1998;228:167–72. doi: 10.1097/00000658-199808000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gigot JF, Jadoul P, Que F, Van Beers BE, Etienne J, Horsmans Y, et al. Adult polycystic liver disease. Is fenestration the most adequate operation for long-term management? Ann Surg. 1997;225:286–94. doi: 10.1097/00000658-199703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swenson K, Seu P, Kinkhabwala M, Maggard M, Martin P, Goss J, et al. Liver transplantation for adult polycystic liver disease. Hepatology. 1998;28:412–15. doi: 10.1002/hep.510280218. [DOI] [PubMed] [Google Scholar]
- 16.Jeyarajah DR, Gonwa TA, Testa G, Abbasoglu O, Goldstein R, Hunsberg BS, et al. Liver and kidney transplantation for polycystic disease. Transplantation. 1998;66:529–32. doi: 10.1097/00007890-199808270-00019. [DOI] [PubMed] [Google Scholar]
- 17.Ueda M, Egawa H, Oike F, Taira K, Uryuhara K, Fujimoto Y, et al. Living-donor liver transplantation for polycystic liver disease. Transplantation. 2004;77:480–1. doi: 10.1097/01.TP.0000110319.60723.31. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson BI, Friman S, Mjornstedt L, Olausson M, Backman L. Liver transplantation for polycystic liver disease – indications and outcome. Transplant Proc. 2003;35:813–14. doi: 10.1016/s0041-1345(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 19.Gigot JF, Legrand M, Hubens G, de Canniere L, Wibin E, Deweer F, et al. Laparoscopic treatment of nonparasitic liver cysts: adequate selection of patients and surgical technique. World J Surg. 1996;20:556–61. doi: 10.1007/s002689900086. [DOI] [PubMed] [Google Scholar]
- 20.Klingler PJ, Gadenstatter M, Schmid T, Bodner E, Schwelberger HG. Treatment of hepatic cysts in the era of laparoscopic surgery. Br J Surg. 1997;84:438–44. [PubMed] [Google Scholar]
- 21.Krahenbuhl L, Baer HU, Renzulli P, Z'graggen K, Frei E, Buchler MW. Laparoscopic management of nonparasitic symptom-producing solitary hepatic cysts. J Am Coll Surg. 1996;183:493–8. [PubMed] [Google Scholar]
- 22.Lai EC, Wong J. Symptomatic nonparasitic cysts of the liver. World J Surg. 1990;14:452–6. doi: 10.1007/BF01658666. [DOI] [PubMed] [Google Scholar]
- 23.Libutti SK, Starker PM. Laparoscopic resection of a nonparasitic liver cyst. Surg Endosc. 1994;8:1105–7. doi: 10.1007/BF00705730. [DOI] [PubMed] [Google Scholar]
- 24.Konstadoulakis MM, Gomatos IP, Albanopoulos K, Alexakis N, Leandros E. Laparoscopic fenestration for the treatment of patients with severe adult polycystic liver disease. Am J Surg. 2005;189:71–5. doi: 10.1016/j.amjsurg.2004.03.011. [DOI] [PubMed] [Google Scholar]