Abstract
Background
Substantial blood loss and the requirement for blood transfusion remain major considerations for hepatic surgeons. We analysed the impact of a systematic protocol aimed at reducing intraoperative blood loss and homologous blood (HB) transfusion associated with hepatic resection.
Methods
Prospective clinical data were collected from 151 elective liver resections performed during the period between 1980 and 1999. Further data directly related to blood loss and anaesthesia were retrospectively collected from the anaesthetic intra-operative record. Strategies implemented in 1991 included preoperative autologous blood donation, low central venous pressure anaesthesia, aprotinin administration, ultrasonic dissection, hepatic vascular inflow occlusion and a Cell Saver. Blood loss and transfusion requirements were studied before and after the implementation of these strategies.
Results
There was no difference in the patient demographics, indications for operation or the scope of resections in the two time periods evaluated. Blood-saving strategies resulted in decreased estimated blood loss (4500 mL vs. 1000 mL p<0.001). In addition, the number of patients requiring transfusion decreased (91.8% vs. 25.5% respectively, p<0.001) and the mean number of units of HB transfusion was lower (I 3.7 vs. 2.3, p<0.001). Morbidity and mortality were also decreased (57.1% vs. 25.5%, p<0.001 and 10.2% and 4.9% p<0.001, respectively). No complications directly referrable to low CVP anesthesia were identified.
Conclusion
Systematic implementation of strategies designed to control blood loss are effective and may reduce morbidity and mortality associated with hepatic resections.
Keywords: blood loss, central venous pressure, aprotinin, ultrasonic dissection, Pringle manoeuvre, Cell Saver, autologous blood transfusion
Introduction
Hepatic resection is the only curative treatment for many liver tumours. Relatively high intraoperative blood loss historically has been a common complication of hepatic resection, frequently resulting in the need for transfusion of blood 1,2,3. In addition, major blood loss and transfusion of HB are associated with postoperative complications and death 4,5,6,7. Numerous strategies to reduce blood loss during hepatic resections have been reported in the literature. However, little is known about the relative importance of the various interventions or the impact of their combined implementation on a surgeon's practice. Recognising the need for minimising blood loss in hepatic resections, we introduced a number of strategies aimed to reduce haemorrhage. This was designated the Minimal Blood Loss (MBL) programme for liver resections and was implemented in 1991. The MBL strategies include preoperative autologous blood (AB) donation and several intraoperative manoeuvres, including the administration of aprotinin, low CVP anaesthesia (≤6 mmHG), temporary hepatic vascular inflow occlusion (Pringle manoeuvre), ultrasonic dissection (Cavitron Ultrasonic Surgical Aspirator, Valleylab, Pfizer Co.), and the use of the Cell Saver to recycle autologous blood intraoperatively.
Aprotinin, a serine protease inhibitor, minimises hepatocellular injury and reduces blood loss. This drug, which is administered intravenously, reduces blood loss through the inhibition of fibrinolytic activity and preservation of the action of platelet binding 8,9. Studies have shown that aprotinin is effective in reducing blood loss in hepatic resection and transplantation 10,11,12. We, and others, have shown that low CVP anaesthesia significantly reduces blood loss during parenchymal transection by keeping the hepatic venous pressure low 13,14,15. In a recent series, Melendez and colleagues showed that low CVP anaesthesia was associated with decreased blood loss and death. The Pringle manoeuvre occludes liver vascular inflow during the transection phase and has been reported to reduce blood loss 16,17. The Cavitron uses high frequency ultrasound waves to dissect liver parenchyma, leaving blood vessels and bile ducts intact so that they can be accurately ligated before transect. Ultrasonic dissection is widely used in hepatic, neurological and urological surgery and represents an advance over the standard crush clamp approach to hepatic parenchymal transection 18,19,20,21. The Cell Saver retrieves the patient's own blood loss intraoperatively, allowing it to be transfused into the patient.
The primary objective of this study is to evaluate the effectiveness of the MBL strategy in reducing blood loss and to assess the relative importance of the various components of the MBL strategy. A secondary objective was to study the impact of the autologous blood donation programme on homologous blood transfusion requirements.
Materials and methods
All hepatic resections in this series were performed by a single surgeon (SSH). Clinicopathological data on all patients were captured in a prospectively collected database, and a chart review was conducted on all 151 cases to ensure completeness and accuracy. Two groups of patients are described: the pre-MBL group (1980–90; n=49) and the MBL group (1991–99; n=102). In the era before the institution of the MBL policy, liver resections were done without the regular implementation of these strategies. Importantly, CVP was often maintained at a high level in anticipation of blood loss, and parenchymal transection was performed with a finger fracture technique. In the MBL era, CVP was routinely maintained ≤6.0 mmHg by restricting the patient's intravenous fluids during parenchymal transection. Neither positive end expiratory pressure (PEEP) nor intermittent positive pressure (IPP) ventilation was used, so that ventilation did not have an appreciable impact on CVP measurement. Aprotinin administration in the MBL era involved a 2 million unit bolus at the start of hepatic transection, followed by a 0.5 million units every hour, up to 4–5 million units in total. Transfusion of AB and HB includes all units of blood transfused intraoperatively and postoperatively until discharge.
Statistical Analysis
We examined estimated blood loss (EBL) in the group with no MBL strategy and the group with the MBL, using a univariate analysis. MBL strategies (Pringle, Cavitron, CVP, aprotinin, Cell Saver) were included in a regression model, along with potential confounding factors including operative time (ORT), number of liver lesions, size of largest lesion, number of segments resected, age, sex and preoperative liver function. The primary end point was EBL. Similarly, stepwise multiple linear regression was used to determine predictors of HB transfusion requirements. The impact of the MBL strategy and the AB program on HB transfusion requirements was determined.
Results
Patient demographic and clinical data are presented in Table 1. Resections were carried out for a variety of indications, including primary and secondary hepatic malignancies and benign hepatic lesions. There were no significant differences between the groups with respect to the complexity of resections. This fact is demonstrated by the similarity in operating time, type of resection and the number of segments resected.
Table 1. Patient demographics and clinical data.
| Total # of patients (%) | Pre-MBL | MBL | p-value | |
|---|---|---|---|---|
| Number of patients | 151(100) | 49 | 102 | |
| Mean age (years) | 60.4 | 59 | 61 | 0.454 |
| Sex | ||||
| Male | 78(51.7) | 24(49.0) | 54(52.9) | 0.651 |
| Female | 73(48.3) | 25(51.0) | 48(47.1) | 0.651 |
| Procedure | ||||
| Major resection | 93(61.6) | 32(65.3) | 61(59.8) | 0.518 |
| Minor resection | 58(38.4) | 17(34.7) | 41(40.2) | 0.518 |
| Indications | ||||
| Hepatoma | 13(8.6) | 4(8.2) | 9(8.8) | 0.880 |
| Metastatic tumour | 110(72.9) | 34(69.4) | 76(74.5) | 0.622 |
| Benign tumour | 28(18.5) | 11(22.4) | 17(16.7) | 0.504 |
By contrast, there is a marked difference in all parameters pertaining to blood loss and transfusion between the two periods (Table 2). Univariate analysis indicated that each of the factors of the MBL was associated with reduced blood loss. There was a significantly lower mean CVP during the transection phase in the MBL group compared to the pre-MBL group (6.4 versus 11.9, p < 0.001). No detectable changes in CVP were observed with normal ventilation. Patients in the MBL group had significantly greater use of the Cavitron, aprotinin, and Cell Saver than patients in the pre-MBL group (p < 0.001). Multiple linear regression modelling indicated that low CVP anaesthesia and aprotinin were the only significant independent predictors of blood loss. Not surprisingly, duration of operation (which is a marker of case complexity) also remained a significant predictor of blood loss (Table 3).
Table 2. Comparison of MBL and Pre-MBL Grops.
| Variable | Pre-MBL (n=49) | MBL(n=102) | p-value |
|---|---|---|---|
| Duration of operation | 353 min | 363 min | 0.727 |
| Type of resection | |||
| Major* | 32(65.3%) | 61(59.8%) | 0.518 |
| Minor | 17(34.7%) | 41(40.2%) | 0.518 |
| Number of segment resected | 3.5 | 3.4 | 0.631 |
| Mean estimated blood loss | 8018±260 mL | 1809±110 mL | <0.001 |
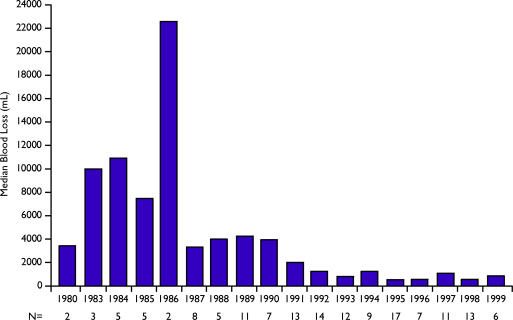
| Median estimated blood loss | 4500 mL | 1000 mL | |
| Mean CVP | 11.9±1.2 mmHG | 6.4±0.3 mmHG | <0.001 |
| Cavitron use | 23(47%) | 97(95%) | <0.001 |
| Aprotinin use | 0(0%) | 91(89%) | <0.001 |
| Cell Saver use | 0(0%) | 21(20.6%) | <0.001 |
| Mean # of units of HB transfused | 13.7±1.8 | 2.3±0.4 | <0.001 |
| Mean # of units of AB transfused | 0 | 0.72±0.2 | <0.001 |
| Mean total # of units transfused | 13.7±1.8 | 3.0±0.4 | <0.001 |
| Patients receiving 1 or more units of HB | |||
| Major resection | 31(96.9%) | 18(29.5%) | <0.001 |
| Minor resection | 14(82.3%) | 8(19.5%) | <0.001 |
| Total | 45(91.8%) | 18(25.5) | <0.001 |
| Morbidity | |||
| Haemorrhage† | 4(8.2%) | 3(2.9%) | 0.159 |
| Bile leak | 6(12.2%) | 12(11.8%) | 0.949 |
| Sepsis | 14(28.6%) | 9(8.8%) | <0.001 |
| Other | 18(36.7%) | 15(14.7%) | 0.002 |
| Overall morbidity‡ | 28(57.1%) | 26(25.5%) | <0.001 |
| Mortality (pen-operative) | 5(10.2%) | 5(4.9%) | <0.001 |
*Major resection is defined as 3 or more segments resected.
†Requiring re-operation for haemorrhage.
‡Patients having 1 or more postoperative complications (bleeding, bile leak, sepsis, other).
Table 3. Multiple linear regression model of predictor of estimated blood loss (EBL).
| Predictors | Univariate p-value | Multivariate p-value |
|---|---|---|
| Duration of operation | 0.727 | <0.001 |
| Mean CVP | <0.001 | 0.044 |
| Aprotinin | <0.001 | <0.001 |
| Pringle manoeuvre | <0.001 | 0.412 |
| Cell Saver | 0.001 | 0.669 |
| Cavitron | <0.001 | 0.819 |
| Number of liver lesions | 0.795 | 0.710 |
| Size of largest lesion | 0.766 | 0.651 |
| Number of segments resected | 0.631 | 0.753 |
| Age | 0.454 | 0.502 |
| Sex | 0.651 | 0.294 |
| Pre-operative liver function | 0.638 | 0.145 |
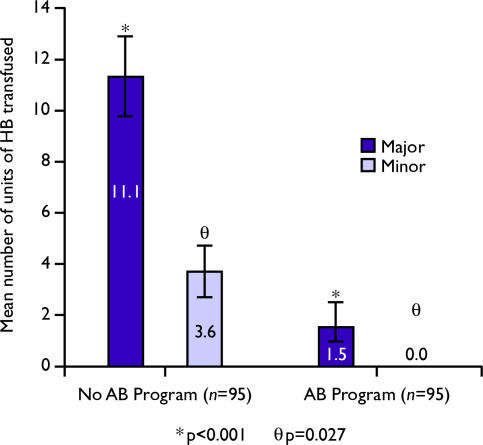
In addition, the overall rate of HB transfusion requirements and the total number of units transfused were significantly lower in the MBL period. The mean amount of HB transfused for the MBL group was 2.3±0.4 units compared to 13.7±1.8 units in the pre-MBL (p < 0.001). The percentage of patients receiving one or more units of HB decreased from 96.9% to 29.5% for major resections (p < 0.001) and from 82.3% to 19.5% for minor resections (p < 0.001). After the initiation of the AB donation programme mean HB transfusions for major resections decreased from 11.1±1.6 units to 1.5±1.1 units (p < 0.001) and for minor resections from 3.6±1.1 units to 0 units (p = 0.027).
Although there were no differences in the rates of sepsis and re-operation for postoperative haemorrhage between the two groups, overall morbidity was significantly lower in the MBL than the pre-MBL group, 25.5% versus 57.1%, (p < 0.001). Moreover, the mortality rate was 4.9% in the MBL group compared to 10.2% in the pre-MBL group (p < 0.001). No patients were identified with serious significant renal dysfunction, myocardial ischemia or air emboli in the era of low CVP anaesthesia.
A subgroup analysis of blood loss and transfusion parameters was conducted for the 20 consecutive patients just before, and the 20 patients following, institution of MBL. This analysis was done to control for surgeon's increasing experience as a factor affecting blood loss with liver resections. Table 4 shows that the first 20 cases after 1991 had a significantly lower EBL than the 20 cases before 1991, but there was no significant difference in duration or the number of segments resected.
Table 4. Comparison of median estimated blood loss (EBL) in 20 cases prior to 1991 and 20 cases after 1991.
| 20 cases Prior to l99l | 20 cases after 1991 | p-value | |
|---|---|---|---|
| Median EBL(mL) | 4100 | 1650 | <0.001 |
| ORT (min) | 359 | 364 | 0.930 |
| Number of segment resected | 2.9 | 2.8 | 0.850 |
ORT = operating room time
Discussion
This study shows that the systematic implementation of strategies aimed to minimise haemorrhage has reduced the estimated blood loss (EBL) in hepatic resections since 1991. Although each factor probably makes an independent contribution to lessen blood loss, it is likely that the combination of these strategies is most important. Like others, we found that aprotinin was useful in reducing blood loss associated with parenchymal transection 10,21. Perhaps more importantly, assiduous control of CVP during the parenchymal transection phase of hepatectomy is a critical determinant of reduced blood loss from hepatic venous branches 11,12,13,15. The maintenance of low CVP often requires a special effort on the part of the anaesthetist, in coordination with the surgeon. Like others, we did not find any complications directly referrable to low CVP anaesthesia 15. Importantly, the benefits accrued from low CVP anaesthesia should be balanced against the potential risk of diminished cardiac output that may occasionally accompany efforts to reduce CVP. At the discretion of the anaesthetist, it was sometimes necessary to insert a Swan-Ganz catheter or to increase CVP to optimise cardiac output. CVP readings are dependent on the positioning of the patient and the level at which the reading is ‘zeroed’. Close attention to such details is important to be certain the absolute CVP reading is relevant. Although not as important in the statistical modelling of EBL, it is likely that the addition of the Pringle manoeuvre, Cell Saver and Cavitron play some role in the success of the MBL strategy.
The duration of operation was a significant predictor of blood loss. Factors that may increase operative duration such as variations in liver anatomy, underlying coagulopathies and extent of resection, may also increase blood loss. Therefore, duration served as a surrogate marker for these factors. It is possible that surgeon experience may affect blood loss, but in our subgroup analysis of 20 cases before and after 1991, there was still a significantly lower amount of blood loss with the implementation of the MBL strategy, suggesting that these strategies caused a marked improvement in blood loss.
Not surprisingly, the institution of MBL was associated with decreased transfusion requirements as a result of decreased blood loss. In addition, the implementation of the AB programme resulted in a further decrease in the need for HB transfusion. This finding is especially relevant in an era in which the risk and cost of transfusion are of increasing importance 22. Perhaps most importantly, the institution of MBL is associated with a decrease in morbidity and mortality rates in patients undergoing resection. Although this may in part be due to increasing surgeon experience, it seems likely that reduced blood loss contributes to these improved outcomes.Figures 1 and 2
Figure 1. .
Median blood loss of patients undergoing liver resections from 1980 to 1999.
Figure 2. .
Impact of the autologous blood (AB) donation programme on homologous blood (HB) transfusion requirements.
The results of this study suggest that liver resections done with careful attention to strategies aiming to reduce blood loss are effective in reducing intraoperative blood loss and HB transfusion requirements. These results underscore the importance of developing a programmatic approach to liver resection that includes cooperation and coordination between anaesthetist and surgeon in both the preoperative period (AB donation) and intraoperative period (aprotinin administration and maintenance of low CVP). Such an approach can lead to dramatic reduction in blood loss and thus obviate one of the major causes of complications and death in hepatic resection.
References
- 1.August DA, Sugarbaker PH, Ottow R, et al. Hepatic resecticn of colorectal metastases. Ann Surg. 1985;201:210–18. doi: 10.1097/00000658-198502000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steele G, Bleday R, Mayer RJ, Lindblad A, Petrelli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol. 1991;9:1105–12. doi: 10.1200/JCO.1991.9.7.1105. [DOI] [PubMed] [Google Scholar]
- 3.Little JM. Hepatic secondaries: minimal tumor and resectable tumor. World J Surg. 1984;8:753–6. doi: 10.1007/BF01655772. [DOI] [PubMed] [Google Scholar]
- 4.Van Ooijen B, Wiggers T, Meijer S, et al. Hepatic resectionfor colorectal metastases in the Netherlands: a multi-institutional 10-year study. Cancer. 1984;70:28–34. doi: 10.1002/1097-0142(19920701)70:1<28::aid-cncr2820700105>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson KR, Steinberg SM, Hughes KS, et al. Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg. 1988;208:679–87. doi: 10.1097/00000658-198812000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younes RN, Rogatko A, Brennan MF. The influence of intra operative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991;214:107–13. doi: 10.1097/00000658-199108000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen CV, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–505. doi: 10.1097/00000658-199210000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt BJ, Cottam S, Segal H, et al. Inhibition of aprotinin of tPA mediated fibrinolysis during orthotopic liver transplantation [letter] Lancet. 1990;336:381. doi: 10.1016/0140-6736(90)91928-4. [DOI] [PubMed] [Google Scholar]
- 9.Lavee J, Savion N, Smolinski A, et al. Platelet protection by aprotinin in cardiopulmonary bypass: electron microstudy. Ann Thorac Surg. 1992;53:477–81. doi: 10.1016/0003-4975(92)90272-6. [DOI] [PubMed] [Google Scholar]
- 10.Lentschener C, Benhamou D, Mercier F, et al. Aprotinin redcues blood loss in patients undergoing elective liver resection. Anesth Analg. 1997;84:875–81. doi: 10.1097/00000539-199704000-00032. [DOI] [PubMed] [Google Scholar]
- 11.Legare G, Roy A, Dagenais M, et al. Reduction of blood loss in orthotopic liver transplantation with the use of aprotinin. Ann Chir. 1996;50:601–5. [PubMed] [Google Scholar]
- 12.Milroy SJ, Cottam S, Tan K, et al. Improved haemodynamic stability with administration of aprotinin during orthotopic liver transplantation. Br J Anaesth. 1995;75:747–51. doi: 10.1093/bja/75.6.747. [DOI] [PubMed] [Google Scholar]
- 13.Hanna SS, Proctor J, Hanna TP. A low central venous pressure reduces blood loss during liver surgery, International Hepatico-pancreatico-Biliary Association 2nd World Congress. In: (Bologna Italy, June 2–6, 1996). Bologna: Monduzzi Editore; 411–15. [Google Scholar]
- 14.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–60. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 15.Melendez JA, Arsian V, Fischer M, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anaesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 16.Arnoletti J, Brodsky J. Reduction of transfusion requirements during major hepatic resection for metastatic disease. Surg. 1999;125:166–71. [PubMed] [Google Scholar]
- 17.Delva E, Camus Y, Nordlinger B, et al. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg. 1989;209:211–18. doi: 10.1097/00000658-198902000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanna SS, Nam R, Leonhardt C. Liver resection by ultrasonic dissection and intraoperative ultrasonography. HPB Surg. 1996;9:121–8. doi: 10.1155/1996/98742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flamm ES, Ranshoff J, Wunchinich D, Broadwin A. Preliminary experience with ultrasonic aspirator in neuro-surgery. Neurosurgery. 1978;2:240–5. doi: 10.1227/00006123-197805000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Addonisio JC, Choudhury MS. Cavitrons in urologic surgery. Uro Clin N A. 1997;13:445–54. [PubMed] [Google Scholar]
- 21.Thomae KR, Mason DL, Rock WA. Randomized blinded study of aprotinin infusion for liver crush injuries in the pig model. Am Surg. 1997;63:113–20. [PubMed] [Google Scholar]
- 22.Mercuriali F, Inghilleri G. Transfusion risks and limitations. Minerva Anestesiol. 1999;65:286–92. [PubMed] [Google Scholar]
- 23.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–9. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]