Abstract
Background
The aim of portal vein embolisation is to induce hyperplasia of normal tissue when resection of a cancerous portion of the liver is contraindicated only by the volume of liver that would remain following operation.
Methods
Eight patients with inoperable liver tumours (3 women and 5 men, median age 69.5 years, 3 colorectal hepatic metastasts,2 choloangiocarcinomas and 3 hepatocellular cancers) were selected for portal vein embolisation. Selected portal branches were occluded with microparticles and coils. Liver volumes were determined by magnetic resonance imaging (MRI) before embolisation and again before operation.
Results
Embolisation was successfully performed in all 8 patients, 7 by the percutaneous-transhepatic route, while one patient required open cannulation of a mesenteric vein. Management was altered in 6 patients who proceded to ‘curative’ resection; projected remaining liver volumes increased (Wilcoxon's matched pairs test p=0.02) from a median of 361 cc to a median of 550 cc; two patients had disease progression such that operation was no longer indicated. In one patient a misplaced coil unintentionally occluded a portal branch to normal liver.
Conclusions
Portal vein embolisation produced appreciable hyperplasia of the normal liver and extended the option of ‘curative’ operation to 6 out of the 8 cases attempted. Complications can occur. The long-term results following operation are unknown.
Keywords: liver neoplasms, imaging, portal vein, embolisation
Introduction
Curative resection is the treatment of choice in localised primary and metastatic liver tumours. Following operation for hepatocellular carcinoma in selected patients, the 5-year survival rate can approach 70% 1 and the 5-year disease-free rate 38% 2. Resection of liver with localised metastatic disease can also produce impressive results for both colorectal neoplasms 3 and non-colorectal malignancies 4.
Estimation of postoperative liver function is problematic as dynamic tests of hepatic clearance of indocyanine green do not give either the maximum amount of liver that can be resected or the functional capacity of the liver that will remain. Futhermore, since the actual volume needed varies between individuals and with the state of the hepatic parenchyma, no absolute minimal volume of liver needed to function can be stated. Up to 60% of a normally functioning liver may be resected 5. Large localised tumours may be considered inoperable if the future liver remnant is too small for adequate postoperative functioning. This problem is amplified if there is accompanying liver parenchymal disease. Whole liver volume and the volume of segments remaining after resection can be accurately measured by either computerised tomography 6 or magnetic resonance imaging 7.
Various efforts have been made to overcome the problem of inadequate postoperative liver volume. For small hepatocellular tumours, transplanting the liver can produce 3-year 8 and 5-year 9 survival figures comparable to or better than those of liver resection. However, transplant options are severely limited by the acute shortage of organs. Furthermore, transplantation is not a suitable option for metastases or large primary tumours.
An alternative strategy is to increase preoperative liver volume by attempting to induce compensatory hypertrophy of the non-involved segments. This manouevre is achieved by inducing ischaemia of the area of the liver to be removed by occluding its blood supply. In this regard embolisation of the hepatic artery has not been effective in hepatocellular carcinoma and only occasionally allows a change in the operative intention for the patient 10. Indeed the surgical outcome has been shown to be worse by some authors following this intervention 11.
Preoperative portal vein embolisation (POPE) has shown more promise. Its main potential benefit is induction of liver hypertrophy to extend the limits of resection. It may also limit centripedal extension of tumour thrombus, prevent dissemination of the tumour cells via the portal vein and possibly (if combined with arterial embolisation) cause complete ischaemic necrosis of the tumour 12. POPE has been successfully performed for primary liver cancers 13 and metastatic liver tumours 14 in both normal and cirrhotic livers 15.
In this paper we present a small experience of POPE in eight patients in liver volumes before ‘curative’ resection with measurements of the changes in liver volumes.
Patients and Methods
Patients were selected for POPE after review by 2 experienced surgeons if they had a liver tumour that was potentially curable by resection and if operation was contraindicated by the size of the future liver remnant calculated from preoperative imaging. Liver volumes and the volumes of uninvolved segments were calculated from staging magnetic resonance imaging, as described by Caldwell 7.
Eight patients with inoperable liver tumours were selected for portal vein embolisation (details summarised in Table 1). There were 3 women and 5 men of median age 69.5 years (range 38–74 years). POPE was performed for colorectal hepatic metastases in 3 patients, choloangiocarcinoma in two and hepatocellular cancer in 3 patients.
Table 1.
| Patient | Age/sex | Type of tumour | Pre-POPE volume | Post-POPE volume | Change in volume % | Tumour location | Approach for POPE | Segments embolised | Operation | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65/f | CAC | 267 | 550 | 106 | Hilar + segment 4 | Ipsilateral then contralateral vein | 4–8 | Liver not operated | Died 2 months after POPE |
| 2 | 38/f | HCC | 578 | 703 | 22 | 4–6 + 8 | Contralateral | 4–8 | Extended right hemihepatectomy | Died 1 1 months after operation |
| 3 | 74/m | HCC | 250 | 602 | 141 | 8 | Ipsilateral | 7 + 8 | Right hemihepatectomy | Died pneumonia postoperatively |
| 4 | 72/f | HCC | 342 | 551 | 61 | 4–6 | Contralateral | 4–8 | Right hemihepatectomy | Died 19 months after operation |
| 5 | 70/m | CHM | 373 | 463 | 24 | 3–6 + 8 | Ipsilateral | 4–8 | Extended right hemihepatectomy | Disease free after 4 months |
| 6 | 67/m | CHM | 442 | 550 | 24 | 2–4 + 8 | Mesenteric | 1–4 + 8 | Extended left hemihepatectomy | Disease free after 5 months |
| 7 | 72/m | CHM | 349 | 500 | 43 | 4–6 + 8 | Contralateral | 5–8 | Extended right hemihepatectomy | Lung relapse after 30 months |
| 8 | 62/m | CAC | 424 | 329 | −22 | Hilar 5 + 6 | Contralateral | 5 + 6 and left branch occlusion | Inoperable at laparotomy | Trial of chemotherapy Died 3 1 months after POPE |
Patient details: f = female, m = male, CAC = cholangiocarcinoma, HCC = hepatocellular carcinoma, CHM = colorectal hepatic metastasis. Volume is given in cc. PRE-POPE VOLUME = projected volume of the liver segments to remain after operation calculated from MRI, POST-POPE VOLUME =Volume of the same segments after embolisation.* = not resected.
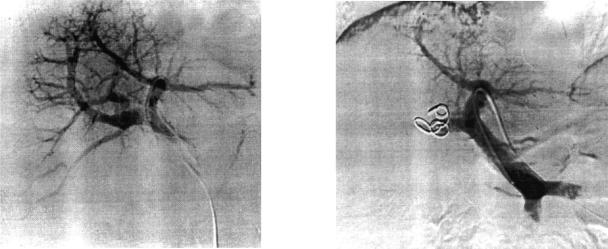
All patients were initially referred for percutaneous embolisation. POPE was performed using a method developed from that previously described by Kinoshita 16. Informed consent was obtained from each patient, and the procedure was performed under light sedation and local anaesthetic. Under ultrasound guidance, a fine needle (22G) was passed percutaneously from the epigastrium through the liver into a branch of the portal vein. Areas of tumour involvement were avoided to prevent needle track dissemination. After securing the punctured portal venous segment with a 018 guidewire, the needle was removed over the wire. A coaxial dilating system (NEFF set, Win Cook UK, St Albans, Herts) was used to dilate the track over the wire. The wire was exchanged through the dilator for a standard 035 guidewire and a 5F vascular sheath was inserted. A curved angio catheter, usually a ‘cobra’ shape, was then used to access the main portal vein and perform preliminary portal venography. From here the catheter was steered into position so that the selected branches could be embolised. Individual segmental vessels were occluded distally with polyvinylalcohol particles (Contour) and proximally with suitably sized coils determined by the vessel diameter (Figure 1). The change in volume of each patient's liver was calculated from sectional magnetic resonance imaging (MRI) obtained before and after embolisation. The first volume measurements were calculated from the initial staging MRI. Follow-up scans were performed at four weekly intervals until hypertrophy of the liver permitted safe operation. A two-tailed Wilcoxon's matched pairs test was used to compare the size of the ‘residual’ liver before and after embolisation. The location of the tumours and the segments embolised are summarised in Table 1.
Figure 1. .
Catheter angiograms showing (a) portal venous anatomy and (b) the occluding coils in the liver and occlusion of the portal branches to the right lobe of the liver after embolisation.
Results
Embolisation was successfully performed in 7 patients by the percutaneous-transhepatic route. One other patient required an open cannulation of the inferior mesenteric vein because a large tumour prevented safe access to the portal vein via the percutaneous route. One patient (no. 1) had POPE attempted via an ipsilateral approach which failed to give access to all the segments selected for embolisation. A contralateral approach one week later successfully completed the embolisation procedure.
Liver volumes and patient outcome
Liver volumes were calculated from MRI obtained in a single breath-hold before and after the procedure in all patients. The projected remaining liver volumes increased from a median of 361 cc (range 250–578 cc) to a median of 550 cc (range 329–703 cc). (Wilcoxon's matched pairs test p = 0.02.)Management was altered in 6 patients (75%) who proceeded to ‘curative’ operation following the induced liver (predominantly left lobe) hypertrophy (Figure 2). The other 2 patients (25%) developed disease progression so that liver resection was no longer indicated. The outcome and survival of patients following POPE are summarised in Table 1.
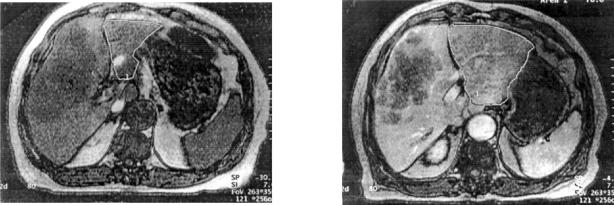
Figure 2. .
Magnetic resonance images showing (a) the tumour in the right lobe and a small left lobe prior to embolisation and (b) the corresponding scan after embolisation showing left lobe hypertrophy. Note the region of the upper pole of the right kidney for orientation of level. The appreciable hypertrophy is noted one month following embolisation.
Complications
Complications occurred in 2 of the 8 patients (25%). There were no deaths due to the procedures and there were no instances of ‘post-embolisation syndrome’. One patient (no. 1) developed a subcapsular haematoma that was successfully managed conservatively. One patient (no. 8), with a hilar bile duct cancer had his left portal branch unintentionally occluded by a misplaced coil that was successfully retrieved, but the left portal branch remained occluded. However, it was noted that flow in the left portal branch vessel was poor before the start of the embolisation. Despite this complication he did not develop abnormal liver function tests or a post-embolisation syndrome, but the left lobe did atrophy. He underwent laparotomy and trial dissection but the tumour was inoperable, invading second order bile ducts on both the right and left sides of the liver.
Discussion
Since the first early experiences of portal vein embolisation in the 1980s 13, many authors have reported its use for hepatocellular carcinomas. Experience of its use in other tumours is very limited, with fewer than 10 cases reported in metastatic cancers 14, 17, 18; these may include repeated publication of different aspects of the same cases. Similarly, experience is limited in bile duct carcinoma 19,20,21.
Various materials can be used to occlude the portal vein including alcohol 22, fibrin glue 23, lipiodol 24, gelfoam, gelatin sponges 25, cyanoacrylate and coils 26. There is no evidence for superior effectiveness of any of them, and each has its exponents. Computed tomography (CT) 6 and magnetic resonance imaging (MRI) 7 can both provide accurate measurements of in vivo liver volumes. To achieve accurate volume measurements from MRI and CT images the scans should be obtained in a single breath-hold. Fast machines are therefore desirable.
In this series the success rate of portal vein embolisation in altering patient managment was 75%. This figure is lower than, but comparable to, other reports: de Baere 25 reports success in 9/10 patients attempted and Elias 27 reports an 82% (23/28) resection rate following POPE.
There were two ‘failures’ in this series associated with disease progression. The principles behind POPE raise oncological concerns. After embolisation an area of liver is made ischaemic, which leads to increased portal blood flow to the other segments of the liver not embolised 28 and possibly a paracrine or endocrine response that eventually results in hypertrophy of the remaining liver as the desired effect. During liver regeneration high levels of growth factors including transforming growth factor-α and hepatocyte growth factor are found, and these mediate the regeneration 29. These growth factors are also tropic to tumour cells, and it is known that liver regeneration per se accelerates the growth of colon tumour cells 30. Indeed Elias 18 has recently observed apparent accelerated metastatic tumour growth in livers undergoing POPE. For these reasons, use of this procedure should be limited to those patients for whom this method offers the only chance of cure. It should be borne in mind that these patients face a bleak alternative, and the same effects are possible after any resection of the liver when regeneration occurs and when micrometastases are present. This fact does not prevent benefit from such resections, but it does seem prudent to minimise the interval between POPE and resection to reduce the risk of progression. Furthermore, this feature of accelerated growth may unmask occult tumour in segments previously thought to be disease-free and may prevent a resection that would not have been of any benefit to the patient. Patients in this series had operations carried out 4–11 weeks following embolisation. Because of the small number of patients world-wide suitable for this intervention, randomised trials of outcome to resolve the oncological concerns are not practical.
A serious complication of POPE that was noted in this series was a misplaced coil that occluded the left portal vein (to the lobe intended to remain). This occlusion did not result in any detectable clinical symptoms or biochemical disturbance, but was associated with disease progression and atrophy of the left lobe. Bleeding causing a subcapsular haematoma and phrenic irritation was also noted on one occasion. Fortunately the bleeding was not severe and did not require intervention. These complications do raise dilemmas over the most appropriate approach to the portal vein. There are three possibilities: 1) An approach on the same side as the tumour, in which the contralateral vein is not compromised, but the needle track may be close to the tumour with the attendant risk of dissemination. In addition the ipsilateral procedure may be technically more difficult because of the need to steer acute angles in the portal system. 2) The contralateral approach which may reduce the risk of tumour seeding, but the vein intended to remain has been traumatised and haemorrhagic complications may be more difficult to manage in liver that should be preserved. 3) It is possible (performed once in this series) to place the catheters into mesenteric vessels surgically, thus avoiding the potential problems of either of the other approaches; this may be the method of choice.
It is of interest to note that tumour progression occurred for each cholangiocarcinoma rather than the hepatocellular cancers or colorectal hepatic metastases, but numbers are too small to attach any meaning to this observation. Although we have provided follow-up data, the length of follow-up is short. Therefore no conclusions about benefit of this intervention can be made at this stage.
In summary, portal vein embolisation produced marked hypertrophy of the normal liver and extended the option of ‘curative’ surgery to 6 out of 8 patients. It appears to be equally effective for primary and metastatic liver tumours in selected patients. Complications can and do occur. An approach to the portal vein via a mesenteric vessel has potential advantages over the percutaneous transhepatic routes. Concerns about the adverse oncological effects of this procedure mean that in the absence of an alternative with good curative or palliative results, its use should be limited to selected cases for whom the prognosis is otherwise hopeless. The shortest time interval between embolisation and operation is desirable rather than maximum hypertrophy. Reports on the long-term results of operation are needed in these patients.
Commentary
In this well-performed clinical study the authors have treated 8 patients with initially inoperable liver tumours by means of portal vein embolisation. Selected portal branches were occluded with microparticles and coils by percutaneous transhepatic route in 7 patients and by open embolisation in one. This type of preoperative therapy should be considered in patients fit for operation but with tumour load beyond resectability. The authors also give advice on different ways of achieving portal vein embolisation, and they state that the transhepatic percutaneous route is the one to be preferred.
One serious problem with this approach is of course the effect on tumour growth, which the authors rightly address. The response to portal vein embolisation is vigorous, and the general response equals that of liver resection in terms of regeneration 1. It induces atrophy in the embolised liver segment. There is ample evidence from the literature that tumour growth is stimulated in experimental systems during the regenerative process, and apparently similar growth stimuli will affect both normal liver and tumour 2. This may be a problem if micrometastases are present in the nonoccluded parts of the liver. However, this effect must be weighed against the bleak outcome for the patient left untreated. One way of diminishing this risk is to keep the length of time between embolisation and operation as short as possible. The authors in this study waited 4–11 weeks. This time interval seemed long enough to induce liver regeneration and short enough not to affect tumour growth. As tumour growth and liver growth seem to be affected by similar stimuli, this is an area of research that should be explored further to increase our knowledge of tumour growth in the liver.
As the authors point out in their discussion, the indications for preoperative embolisation will be limited to only a few patients in each centre, and it is therefore difficult to perform a randomised trial. Nevertheless, there are enough data to recommend that preoperative portal embolisation should be used in selected cases of irresectable liver tumours.
Professor BW Jeppsson
Department of Surgery
Malmö University Hospital
Malmö
Sweden
S-205 02
Commentary
Mr Seymour and his colleagues report here their experience of a small series of patients submitted to portal vein embolization (PVE) before liver resection for primary and metastatic liver tumours, primarily considered as irresectable due to the insufficient size of the future remnant liver.
This series clearly illustrates the two limitations of this technique that might preclude liver resection; (i) technical errors during portal embolisation and (ii) disease progression in the interval before liver resection.
To avoid unintentional contralateral portal emboli sation, the use of an occlusive balloon (1,2) or a different embolic agent (such as glue mixed with lipiodol) allows better fluoroscopic visualisation and better control of the embolisation process (3).
The disease progression during the interval between PVE and liver resection should be prevented by the administration of adjuvant therapy, e.g. arterial chemo-embolisation at least three weeks before PVE in patients with hepatocellular carcinoma (1,2), or adjuvant chemotherapy (such as 5 fluoro-uracil and folinic acid with oxaliplatine) in patients suffering from colorectal liver metastases (2). By using this strategy, we observed a significant decrease of liver-metastatic disease at the time of liver resection six-to-eight weeks later.
The criteria for selecting the patients undergoing PVE are unclear in this series. In fact, the definition of insufficient liver remnant varies between the different published series (1,2,3,4,5). While the relative volume of the remnant liver is the most important criterion, other factors such as active hepatitis B carriage, presence of liver cirrhosis, liver steatosis or steatofibrosis and a previous history of arterial chemo-embolisation or multiple courses of neoadjuvant chemotherapy, should also be taken into consideration. The respective weight of these factors remains difficult to evaluate.
Since the complication rate of PVE is negligible in our initial personal experience of 22 patients (6), we are now performing PVE for all patients with a future liver remnant volume less than 25% of the whole liver in the absence of the above risk factors. The volume of the residual functional liver parenchyma should always be calculated by subtracting the volume of liver tumours. In addition, the liver volume measurement should be performed by the same type of radiological investigation (preferably magnetic resonance imaging technique in our experience) due to the imperfect reproducibility of such calculation with different imaging techniques.
Finally, there is no doubt that PVE is a major advance in the management of the patient with extensive malignant primary or metastatic liver diseases. A multidis-ciplinary approach with interventional radiologists allows optimal care for the patients.
Jean-François Gigot
Department of Digestive Surgery
Pierre Goffette
Department of Imaging Techniques
Saint-Luc University Hospital
Universite Catholique de Louvain (UCL)
Hippocrate Avenue, 10
1200 Brussels, Belgium
References
- 1.Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221:291–8. doi: 10.1097/00000658-199503000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takenaka K, Kawahara N, Yamamoto K, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71–6. doi: 10.1001/archsurg.1996.01430130073014. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 4.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187:487–93. doi: 10.1016/s1072-7515(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 5.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–81. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 6.Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Ued. 1979;90:185–7. doi: 10.7326/0003-4819-90-2-185. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell SH, de Lange EE, Gaffey MJ, et al. Accuracy and significance of pretransplant liver volume measured by magnetic resonance imaging. Liver Transpl Surg. 1996;2:438–42. doi: 10.1002/lt.500020606. [DOI] [PubMed] [Google Scholar]
- 8.Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424–32. doi: 10.1097/00000658-199803000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangro B, Herraiz M, Martinez-Gonzalez MA, et al. Prognosis of hepatocellular carcinoma in relation to treatment: a multi-variate analysis of 178 patients from a single European institution. Surgery. 1998;124:575–83. [PubMed] [Google Scholar]
- 10.Elias D, Lasser P, Rougier P, Ducreux M, Bognel C, Roche A. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–19. [PubMed] [Google Scholar]
- 11.Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'Eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–6. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka T. Experimental studies of intrahepatic portal vein embolization and embolic materials. Nippon lgaku Hoshasen Gakkai Zasshi — Nippon Acta Radioligca. 1989;49:593–606. [PubMed] [Google Scholar]
- 13.Matsuoka T, Nakatsuka H, Kobayashi N, et al. Portal vein embolization for hepatoma with lipiodol-fibrin adhesive mixture] Nippon lgaku Hoshasen Gakkai Zasshi – Nippon Acta Radiologica. 1984;44:1411–13. [PubMed] [Google Scholar]
- 14.Soyer P, Roche A, Elias D, Levesque M. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology. 1992;184:695–7. doi: 10.1148/radiology.184.3.1509051. [DOI] [PubMed] [Google Scholar]
- 15.Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109–15. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–8. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 17.Yamakodo K, Takeda K, Matsumura K, et al.Regeneration of the un-embolized liver parenchyma following portal vein embolization J Hepatol 27:871–80. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Roche A, Ducreux M, Leclare J, Lasser P. During liver regeneration following right portal embolisation the growth rate of liver metatstases is more rapid than that of liver parenchyma. Br J Surg. 1999;86:907–11. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagino M, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolisation before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677–81. doi: 10.1016/s0039-6060(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 20.Vogl TJ, Balzer JO, Dette K, et al. Initially unresectable hilar cholangiocarcinoma: hepatic regeneration after transarterial embolisation. Radiology. 1998;208:217–22. doi: 10.1148/radiology.208.1.9646816. [DOI] [PubMed] [Google Scholar]
- 21.Makuuchi M, Kosuge T, Lygidakis NJ. New possibilities for major liver surgery in patients with Klatskin tumors or primary hepatocellular carcinoma old problem revisited. Hepatogastroenterology. 1991;38:329–36. [PubMed] [Google Scholar]
- 22.Ogasawara K, Uchino J, Une Y, Fujioka Y. Selective portal vein embolization with absolute ethanol induces hepatic hypertrophy and makes more extensive hepatectomy possible. Hepatology. 1996;23:338–45. doi: 10.1053/jhep.1996.v23.pm0008591861. [DOI] [PubMed] [Google Scholar]
- 23.Nagino M, Nimura Y, Kamiya J, Kondo S, Kanai M. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200:559–63. doi: 10.1148/radiology.200.2.8685357. [DOI] [PubMed] [Google Scholar]
- 24.Liu K, Lu J, Tan W. [Portal vein supply and embolization therapy for hepatocellular carcinoma]. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal] 1995;75:403–5, 445. [PubMed] [Google Scholar]
- 25.Baere de T, Roche A, Vavasseur D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993;188:73–7. doi: 10.1148/radiology.188.1.8511321. [DOI] [PubMed] [Google Scholar]
- 26.de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–91. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Debaere T, Roche A, Bonvallot S, Lasser P. Preoperative selective portal vein embolizations are an effective means of extending the indications of major hepatectomy in the normal and injured liver. Hepatogastroenterology. 1998;45:170–7. [PubMed] [Google Scholar]
- 28.Goto Y, Nagino M, Nimura Y. Doppler estimation of portal blood flow after percutaneous transhepatic portal vein embolization. Ann Surg. 1998;228:208–13. doi: 10.1097/00000658-199808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killion R, Jr, Fiddler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer and Matastasis Reviews. 1999;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 30.Gutman M, Singh RK, Price JE, Fan D, Fidler IJ. Accelerated growth of human colon cancer cells in nude mice undergoing liver regeneration. Invasion and Metastasis. 1994;14:362–71. [PubMed] [Google Scholar]
- 31.Rozga J, Jeppsson B, Bengmark S. Portal branch ligation in the rat. Reevaluation of a model. Am J Pathol. 1986;125:300–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Rozga J, Tanaka N, Jeppsson B, Hägerstrand I, Bengmark S. Tumor growth in liver atrophy and growth. An experimental study in rats. Eur Cancer Clin Oncol. 1985;21:135–40. doi: 10.1016/0277-5379(85)90210-x. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–8. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 34.Lee CK, Kinoshita H, Hirohashi K, et al. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109–15. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- 35.de Baere T, Roche A, Elias D, et al. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–91. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 36.Azoulay D, Castaing D, Smail A, et al. Resection of nonre-sectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–6. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche A, Lasser P, de Baere T, Elias D. L'embolisation portale preoperatoire un moyen efftcace pour hypertrophier le fole sain et élargir les indications des resections hepatiques. Chirurgie. 1998;123:67–73. doi: 10.1016/s0001-4001(98)80041-x. [DOI] [PubMed] [Google Scholar]
- 38.Goffette P, Lerut J, Laterre PF, Gigot JF. Preoperative portal vein embolization for extension of hepatectomy indications. Vase Intervent Radiol. 2000;11:265(A). [Google Scholar]