Abstract
Staging systems are key to predict the prognosis of patients with cancer, to stratify the patients according to prognostic variables in the setting of clinical trials, to allow the exchange of information among researchers, and finally to guide the therapeutic approach. The current knowledge of the disease, however, prevents recommendation of a staging system that can be used world-wide. The conventional staging systems for hepatocellular carcinoma (HCC), such as the Okuda stage or the TNM stage have shown important limitations in classifying patients. Several new systems have been proposed recently, and only three of them have been validated at this point. The BCLC staging classification links the stage of the disease to a specific treatment strategy. The JIS score has been proposed and used in Japan, although it needs Western validation. The CLIP score is used in patients with advanced tumors. Several reasons explain the difficulty in identifying a world-wide system. First, HCC is a complex neoplasm inserted on a pre-neoplastic cirrhotic liver, and thus variables of both diseases leading to death should be taken into account. Second, the disease is very heterogeneous around the world, and this reflects different underlying epidemiological backgrounds and risk factors. Third, HCC is the sole cancer treated by transplantation in a small proportion of patients. Fourth, only around 20% of the cases are currently treated by surgery, thus precluding the wide use of pathology-based systems, such as TNM. Finally, the potential relevance of a molecular signature identified in terms of outcome prediction is unknown, and further research is needed to obtain this valuable biological information that may aid in classifying the patients.
Keywords: Hepatocellular carcinoma, natural history, staging system, prognosis, treatment, randomized controlled trials, resection, liver transplantation, percutaneous ablation, meta-analysis, chemoembolization, BCLC staging classification, JIS score
Introduction
Hepatocellular carcinoma (HCC) is a major health problem worldwide. It is the fifth most common neoplasm in the world, with more than half million new cases yearly 1. The incidence of HCC rose in the last decade. In the USA, the incidence of HCC is expected to increase over the next two decades, equalling that currently experienced in Japan 2. HCC is now the leading cause of death among cirrhotic patients 3.
Risk factors
Hepatocellular carcinoma develops in a cirrhotic liver in 80% of cases, and this preneoplastic condition is the strongest predisposing factor 4. Hepatitis B virus (HBV) infection is the main risk factor in Asia and Africa. Chronic carriers have a 100-fold relative risk for developing HCC, with an annual incidence rate of 2–6% in cirrhotic patients 5. Afiatoxin B1 intake further enhances the risk. In Western countries and Japan, hepatitis C virus (HCV) infection is the main risk factor, together with other causes of cirrhosis 4,6. Around 20–30% of the estimated 170 million HCV-infected individuals worldwide will develop cirrhosis. Once cirrhosis is established, the annual incidence of HCC is 3–5%, and one-third of these individuals will develop an HCC over their lifetime 4.
Natural history
The natural history of this neoplasm is not completely known. First, there are no data regarding the timescale of the whole hepatocarcinogenetic process, which evolves from the onset of the neoplasm until the time in which it is diagnosed in the setting of surveillance programmes. Second, early diagnosis of HCC still relies on pathological data rather than molecular data, and thus the accuracy of differentiating premalignant lesions and early neoplasm is still ill defined. Finally, once diagnosis is established, the prognosis of patients will vary according to the evolutionary stage at which the neoplasm is diagnosed and the treatment received.
Survival of HCC patients has improved because of the advancement of the time of diagnosis and the increase in therapeutic efficacy. The prognosis of HCC was dismal two decades ago 7. Nowadays, however, 40% of HCC patients may receive curative treatments 4,8. These treatments are assumed to improve the natural history of the disease. The best survival outcome without treatment is 65% at 3 years for Child-Pugh class A patients with single tumors 9, whereas after radical therapies survival reaches 70% at 5 years 4,8. The natural course of advanced stage HCC is better known. The 1- and 2-year survival rates of untreated patients within 25 RCTs were 10–72% and 8–50%, respectively 10. Overall, two groups of patients with nonsurgical HCC have been identified: patients at the intermediate stage (asymptomatic tumors) show a 3-year survival rate of 50%, compared with 8% of patients at the advanced stages 11. Patients at terminal stages survive <6 months 7.
The process of carcinogenesis
The molecular pathogenesis of HCC is complex 12,13. The most accepted hypothesis describes a step-by-step process through which external stimuli induce genetic alterations in mature hepatocytes leading to cell death and cellular proliferation (regeneration). In the progression of chronic inflammation to fibrosis and cirrhosis, the up-regulation of mitogenic pathways leads to the production of monoclonal populations. These populations harbor dysplastic hepatocytes as a result of altered gene expression, telomerase erosions and even chromosome aberrations. This process may last 10–30 years 13. At this point, proliferation may be detected in isolated groups of cells, resulting in foci of small cell dysplasia or, more frequently, surrounded by a fibrotic ring resulting in low-grade dysplastic nodules (LGDN) or high-grade dysplastic nodules (HGDN) 14. These are the major preneoplastic entities, although HCC may also arise from isolated small dysplastic cells, nonconforming clear hepatic nodules, or even from progenitor cells, which may develop mixed tumors. HGDN are currently considered truly preneoplastic lesions, and may develop into malignant tumors in 30% of cases over a period of 1–5 years 15,16.
Diagnosis and prognosis of early HCC
The panel of experts on HCC of the European Association for the Study of the Liver (EASL) has recently proposed diagnostic criteria for HCC that rely either on conventional histologic characteristics or on noninvasive criteria 17. Nodules <2 cm in size should be diagnosed by means of conventional pathological criteria. Accuracy of fine-needle biopsy directly depends on the size of the nodule, and ranges between 50% and 70% in small HCC of <2 cm in diameter 19. If nodules are <1 cm, only half of them will correspond to HCC, and it is almost impossible to correctly diagnose it with the current diagnostic tools. Differentiation of early well-differentiated HCC from preneoplastic lesions is a histopathologic challenge, and molecular markers are awaited in this setting 14,18. Conversely, for nodules >2 cm in the setting of liver cirrhosis, HCC may be confidently diagnosed by the coincidental findings of two imaging techniques (ultrasonography, spiral CT or magnetic resonance imaging) showing arterial hypervascularization, or by a single positive imaging technique associated with alfa-fetoprotein (AFP) >400 ng/ml 17.
Survival of patients with early HCC in referral liver units may achieve 50–70% at 5 years after resection, liver transplantation or percutaneous treatments 4,8. In these cases, it is assumed that therapies actively modify the natural course of the disease. These outcomes are the result of applying the so-called treatment-dependent variables in the selection of candidates. In summary, these variables are single tumors with a very well preserved liver function (no portal hypertension, normal bilirubin) for resection, single tumors ≤5 cm or three nodules ≤3 cm for liver transplantation, and single tumors ≤3 cm in Child-Pugh A patients for percutaneous treatments 4,20. Percutaneous treatments provide good results, but are unable to match the outcomes achieved with surgery 20.
Recurrence is the major drawback of potentially curative treatments. This is due to the fact that cancer invasion and dissemination may occur in some tumors <2 cm, although others behave as the carcinoma-in- situ entity 18. Kojiro et al. analysed 106 resected HCC ≤ 2 cm and distinguished the so-called indistinct type (mean size 12 mm) without local invasiveness, from the distinct nodular type (mean size 16 mm) that showed local invasiveness. In the latter type, local metastases surrounding the nodule were found in 10% of cases, and microscopic portal invasion in up to 25%. The metastatic potential of the so-called ‘early HCC’ has been confirmed by gene expression assessment through microarrays 21.
Intermediate-advanced HCC
Prognosis of HCC was assumed to be poor when radical treatments were not feasible. This assumption was based on data reported in studies describing series of untreated HCC patients diagnosed at different evolutionary stages, the median survival figures being <1 year 7. These figures have also been recently reproduced when analysing survival estimates gathered from population-based cancer registries 22. However, most of the patients were recruited retrospectively >10 years ago, when regular screening was uncommon, the imaging techniques were less accurate, and the medical management was less effective than nowadays. Thus, the outcome of untreated HCC has dramatically changed. Nowadays, the natural history of HCC at intermediate-advanced stages can be assessed with recent data obtained from patients randomized to the untreated arm in the setting of randomized controlled trials (RCTs). More than 20 RCTs have been published, including an untreated arm of conservative management 10. The 1- and 2-year control survival rates in these trials were 10–72% and 8–50%, respectively. The wide disparity of these figures reflects the heterogeneity of the population considered as merely with ‘unresectable HCC’, that were suitable for other therapies in the setting of RCT. The completion of two RCTs including a ‘no treatment’ arm allowed us to recruit a cohort of 102 untreated HCC patients, who had been prospectively followed 23. Their survival was 54%, 40% and 28%, at 1, 2 and 3 years, respectively, and the best predictors of survival were the presence of cancer-related symptoms (Performance Status Test (PST) = 1–2 or constitutional syndrome) and the identification of an invasive pattern evidenced by the presence of vascular invasion or extrahepatic spread. Thereby, two subgroups with a markedly different life expectancy can be identified among patients in an intermediate evolutionary stage. Patients in a truly intermediate stage (asymptomatic patients without a tumoral invasive pattern) showed a 1-, 2-, and 3-year survival rate of 80%, 65%, and 50%, respectively, compared with those patients at an advanced stage (at least one adverse prognostic factor), their corresponding values being 29%, 16%, and 8%, respectively 23.
End-stage HCC
Patients with end-stage disease are characterized by presenting Okuda stage III, or Performance Status of 3–4, that reflects a severe tumor-related disability. Similarly, advanced tumors in Child-Pugh C patients also account for a very poor prognosis. We have recently reported a 5% 6-month survival rate in Child-Pugh C patients presenting with spontaneous bacterial peritonitis and advanced tumors 24.
Staging systems in HCC
Cancer staging should serve to select the appropriate primary and adjuvant therapy, to estimate the prognosis, and also to assist in the evaluation of the results of treatment, and to exchange information without ambiguity 25. In oncology, the prognosis of patients with solid tumors is solely related to tumor stage, and other co-factors such as age or histologic grade are only seldom considered. However, HCC patients constitute a particular case, as cirrhosis underlies the neoplasm in most individuals and thus, their outcome is related to these two entities, which simultaneously determine the applicability and efficacy of treatments. Accordingly, prognostic modeling in HCC patients is highly complex. The EASL panel of experts recommended the consideration of four related aspects: tumor stage, degree of liver function impairment, general condition of the patient, and treatment efficacy 17. Survival of early stage patients is modified by treatment and thus, prognostic prediction has to include treatment-related variables. At more advanced stages, treatment might not be identified as a relevant survival predictor and the use of a single prognostic model for all patients may appear adequate. Nowadays, experts in HCC management may choose among eight different staging systems 7,11,26,27,28,29,30,31 (Table I), none of them with universal acceptance 26. The variables included in each classification are different, reflecting the heterogeneous methodology used, and the population used to construct the models (Table II). Three of them – BCLC, CLIP and JIS score – have been validated in different cohorts of patients (Table III), whereas other studies have not identified any superior system 32,33,34,35,36,37. Therefore, a consensus staging classification for HCC is needed.
Table I. Staging systems in hepatocellular carcinoma.
| Classification | Type | Stages | Reference |
|---|---|---|---|
| Okuda stage | System 3 | Stage I, II, III | 7 |
| French | Score 3 | A: 0 points; | 26 |
| B: 1–5 points; | |||
| C: ≥6 points | |||
| CLIP | Score 7 | 0, 1, 2, 3,4, 5, 6 | 27 |
| BCLC staging | Staging 5 | 0: Very early | 11 |
| A: Early | |||
| B: Intermediate | |||
| C: Advanced | |||
| D: End-stage | |||
| CUPI | Score 3 | Low risk: score ≤1 | 28 |
| Intermediate: score 2–7 | |||
| High: score ≥8 | |||
| TNM staging | System 3 | Stage I, II, III | 29 |
| JIS | Score 4 | Stage I, II, III, IV | 30 |
| ER | System 2 | ER wild-type | 31 |
| ER variant |
Table II. Prognostic variables used in the staging systems in hepatocellular carcinoma.
| Variables | |||
|---|---|---|---|
| Classification |
Tumor stage |
Liver function |
Health status |
| Okuda stage 7 | 50% liver involvement | Bilirubin | – |
| Albumin | |||
| Ascites | |||
| French 26 | Portal invasion | Bilirubin | Karnofsky |
| AFP | Alkaline phosphatase | ||
| CLIP 27 | Portal invasion | Child-Pugh | – |
| </ > 50% liver involvement | |||
| AFP | |||
| BCLC 4,11 | Portal invasion | Child-Pugh | PST |
| Metastases | Portal hypertension | ||
| Morphology | Bilirubin. | ||
| Okuda | |||
| CUPI 28 | TNM | Ascites | Symptoms |
| AFP | Bilirubin | ||
| Alkaline phosphatase | |||
| TNM 29 | Morphology | Fibrosis | – |
| Vascular invasion | |||
| Metastases | |||
| JIS score 30 | TNM | Child-Pugh | – |
| ER 31 | Estrogen receptor | – | – |
Table III. Comparison of staging systems for HCC.
| Authors | Journal | Year/Ref | Country | Comparison | Best | Conclusion |
|---|---|---|---|---|---|---|
| Cillo et al. | J Hepatol | 2004 32 | Italy | 5 systems | BCLC | Validation BCLC |
| Villa et al. | J Clin Oncol | 2003 31 | Italy | 5 systems | ER | Proposal ER |
| Rabe et al. | Eur J Gastroenterol Hepatol | 2003 33 | Germany | 5 systems | None | – |
| Leung et al. | Cancer | 2002 28 | China | 4 systems | CUPI | Proposal CUPI |
| Giannini et al. | J Intern Med | 2004 34 | Italy | 4 systems | None | – |
| Ueno et al. | Hepatology | 2002 35 | Japan | 3 systems | CLIP | Validation CLIP |
| Farinati et al. | Cancer | 2000 36 | Italy | 3 systems | CLIP | Validation CLIP |
| Levy and Sherman | Gut | 2002 37 | Canada | 3 systems | CLIP | Validation CLIP |
| Kudo et al. | J Gastroenterol | 2003 30 | Japan | 2 systems | JIS | Proposal JIS score |
Okuda stage
The Okuda classification 7 has been widely applied in HCC patients in the last decade. It includes parameters related to the liver functional status – albumin, ascites, bilirubin – and to the tumor stage – more or less than 50% of liver area involved. This classification properly stratified patients when most of them were diagnosed at an advanced/symptomatic stage. It is useful to identify end-stage patients (Okuda stage III), that should not be included in therapeutic trials to assess the potential benefits of new therapeutic agents due to their grim prognosis. Nowadays, however, the time of diagnosis has been advanced and thus, this classification is not adequate to stratify patients prior to radical or palliative therapies, even when dividing Okuda stage I patients into two subgroups according to tumor size. When compared with modern staging systems, it has been shown to have lower predictive capacity 32,33,34,35,36.
French classification
The French classification 26 was constructed with the analysis of 761 HCC patients, among which 47% received specific treatments. This classification combines five variables in a score system that stratifies patients in three stages. Survival of these stages at 2 years was of 51%, 16%, and 3%, respectively, and reflects the fact that this cohort mostly included patients at advanced stages. A recent comparison with other staging systems has shown that it has limited prognostic capacity in patients with early HCC 32.
CLIP score
The Cancer of the Liver Italian Program (CLIP) score 27 was constructed in a retrospective study and validated by the authors and other groups 35,36,37. This score combines four variables that provide a seven-stage classification system. It has been compared with Okuda stage and TNM stage with better discriminatory power. Asian groups have reported survival rates clearly different to the original authors, thus compromising their external validation 35. It is also limited by the fact that it does not serve to select the appropriate therapy for each patient.
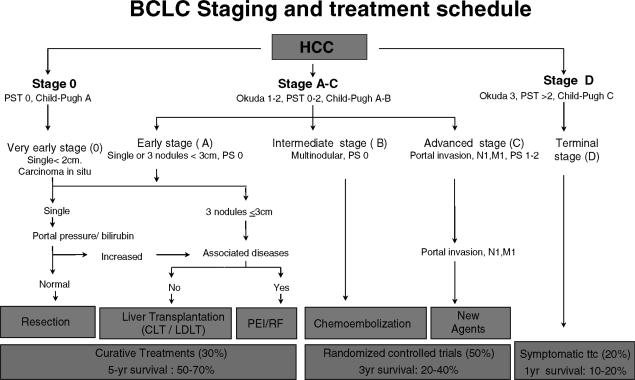
BCLC staging system.
The Barcelona-Clínic Liver Cancer (BCLC) staging system 4,8,11 was constructed on the basis of the results obtained in the setting of several cohort studies and RCTs by the Barcelona group. This proposal is not a scoring system as it derives from the identification of independent prognostic factors in the setting of several studies, conforming a staging classification. This classification uses variables related to tumor stage, liver functional status, physical status, and cancer-related symptoms, and links the four stages described with a treatment algorithm (Figure 1). In brief, patients at stage 0 with very early HCC are optimal candidates for resection. Patients at stage A with early HCC are candidates for radical therapies (resection, liver transplantation or percutaneous treatments). Patients at stage B with intermediate HCC may benefit from chemoembolization. Patients at stage C with advanced HCC may receive new agents in the setting of RCT, and patients at stage D with end-stage disease will receive symptomatic treatment.
Figure 1. .
Barcelona-Clinic Liver Cancer (BCLC) staging classification and treatment schedule. (Adapted from Llovet JM et al., Lancet 2003 4.)
It has been suggested that this classification is best suited for treatment guidance, and particularly to select early stage patients who could benefit from curative therapies 38. In that sense, it has recently been validated as the best staging system in a cohort of patients with early HCC 32.
CUPI score
Investigators in Hong Kong described a staging system analysing their experience in 926 patients, most of them with HBV-related cirrhosis 28. The Chinese University Prognostic Index (CUPI) considers six predictive variables, and divides patients into three stages. The authors estimate that this classification has better estimation of survival than CLIP score and Okuda stage, although its discriminatory power in early stages is questionable, as the best 1-year survival was around 50%.
TNM stage
The conventional TNM system, which only contains variables related to tumor stage, has been mostly tested in the surgical setting, and showed poor prognostic prediction in HCC patients undergoing either resection 39 or transplantation 40. Based on the results of a series of 557 patients who underwent resection 29, a recent modification has been proposed, including tumor stage and presence of fibrosis. The new four-stage system may improve the stratification of resected tumors, even though it is controversial whether they will apply to nonsurgical patients. It has been endorsed by the American Joint Committee on Cancer (AJCC).
JIS staging
The Japan Integrated Staging (JIS) is a new score system that includes two previous classifications: the TNM endorsed by the Union Internationale Contre le Cancer (UICC) 41, mostly applied in Japan, and the Child-Pugh classification. It lacks external validation in Western countries.
Critical appraisal of HCC classifications and future prospects
There is no doubt that the classical staging systems have already been improved. The Okuda staging and the Child-Pugh classification might be used as a part of any new clinical staging system, but should no longer be used alone. Attempts to improve the classification and prognosis prediction of HCC are still evolving, and there is no agreement on the best staging that can be recommended worldwide 17. The heterogeneous survival figures described for the best stages (3-year survival from 80% 11 to 25% 28) reflect that some studies include mostly advanced cases with a minor number of effectively treated patients. In these studies, treatment-related variables might not be identified as a relevant survival predictor and the use of the same set of variables for all patients may appear adequate. Conversely they face the same difficulty in early cases, as prognostic modeling for early HCC has specific requirements 26,27,28. The CUPI, CLIP, and French staging systems have been constructed with patients at advanced stages. They use rough descriptions of tumor stage that are not in accordance with the predictive value of tumor size and multicentricity. For instance, the CLIP score classifies the tumor burden as above/below 50% of liver involvement, thus making it impossible by definition to identify patients at early stages. The new TNM according with the AJCC has only internal validation, and is based on series of patients undergoing resection 29, as is the case with the seminal paper proposing JIS classification 30. Pathologic information is needed in all cases, this representing a limitation for wide clinical use.
The BCLC staging system has been validated by a surgically oriented European group 32. This study includes the widest comparison among staging systems, in comparison with other retrospective studies in which the limited collection of data impairs the ability to test all the systems available. The BCLC staging system may discriminate patients at early stages, and guide the treatment strategy. More recently, new systems have appeared, suggesting a stronger discriminatory power when compared with published ones 31. Even some societies have endorsed one of the systems 42, with controversial acceptance 43,44.
Our current level of knowledge prevents recommendation of a staging system to be used worldwide. HCC is a complex neoplasm, in most cases on a background of a preneoplastic damaged liver. Both diseases may lead to death. In addition, unlike breast cancer 45 and lymphoma 46, no clear biologic/genetic markers have been shown to have prognostic value in HCC. In that sense, several human malignant tumors have recently been classified with respect to their prognostic outcome or response to treatment according to gene expression profile identified through micro-array technology. Investigators have developed gene expression-based classifications for breast cancer, non-Hodgkin's B-cell lymphoma, leukemia, lung carcinoma, prostate cancer, bladder cancer, and melanoma. Thus, molecular markers are needed in HCC. However, to be clinically useful, the molecular classification should be incorporated into a staging scheme, which effectively separates patients into groups with homogeneous prognosis and response to treatment, and thus serves to aid in the selection of appropriate therapy. The potential relevance of a molecular signature identified in terms of outcome prediction should ultimately be tested in large cohorts of patients and analysed together with well-known clinical variables, as has been done recently for breast cancer 47.
Acknowledgements
Josep M Llovet is a recipient of a contract from Programa “Ramon y Cajal” (IDIBAPS, Ministerio de Ciencia y Tecnología). This review was partially supported by a grant from AGAUR 2003BEAI00138 (Generalitat de Catalunya) and Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias 2002–2005, PI020596) M.V. is a recipient of a Grant ‘Premi Fin de Residencia Emili Letang’ (Hospital Clinic, Barcelona).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, et al. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:15584–9. doi: 10.1073/pnas.242608099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, et al. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986;90:263–7. doi: 10.1016/0016-5085(86)90919-4. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, et al. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004–6. doi: 10.1016/s0140-6736(89)91015-5. [DOI] [PubMed] [Google Scholar]
- 7.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Haregawwa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 9.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–7. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 12.Buendia MA. Genetics of hepatocellular carcinoma. Semin Cancer Biol. 2000;10:185–200. doi: 10.1006/scbi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 13.Thorgeirsson S, Grisham J. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–46. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 14.Theise N, Park Y, Kojiro M. Dysplastic nodules and hepatocarcinogenesis. Clin Liv Dis. 2002;6:497–512. doi: 10.1016/s1089-3261(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 15.Seki S, Sakaguchi H, Kitada T. Outcomes of dysplastic nodules in human cirrhotic liver: a clinicopathological study. Clin Cancer Res. 2000;6:3469–73. [PubMed] [Google Scholar]
- 16.Borzio M, Fargion S, Borzio F. Impact of large regenerative, low grade and high grade dysplastic nodules in hepatocellular carcinoma development. J Hepatol. 2003;39:208–14. doi: 10.1016/s0168-8278(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Christensen E, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona- EASL Conference. J Hepatol ;35. 2000;2001:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Kojiro M. Focus on dysplastic nodules and early hepatocellular carcinoma: an eastern point of view. Liver Transpl. 2004;10:S3–S8. doi: 10.1002/lt.20042. [DOI] [PubMed] [Google Scholar]
- 19.Borzio M, Borzio F, Macchi R, Croce AM, Bruno S, Ferrari A, et al. The evaluation of fine-needle procedures for the diagnosis of focal liver lesions in cirrhosis. J Hepatol. 1994;20:117–21. doi: 10.1016/s0168-8278(05)80477-5. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;39:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 21.Ye QH, Qin LX, Forgues M. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–23. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 22.El Serag HB, Mason A, Key CH. Trends in survival of patients with hepatocellular carcinoma between and 1996 in United States 1997. Hepatology. 2001;33:62–5. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso MC, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 24.Llovet JM, Moitinho E, Sala M, Bataller R, Rodríguez-Iglesias MP, Castells A, et al. Prevalence and prognostic value of hepatocellular carcinoma in cirrhotic patients with spontaneous bacterial peritonitis. J Hepatol. 2000;33:423–9. doi: 10.1016/s0168-8278(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 25.Fleming I. AJCC/TNM Cancer staging, present and future. J Clin Oncol. 2001;77:233–6. doi: 10.1002/jso.1101. [DOI] [PubMed] [Google Scholar]
- 26.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. J Hepatol. 1999;31:133–41. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 27.CLIP. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000;31:840–5. [DOI] [PubMed] [Google Scholar]
- 28.Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–69. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 29.Vauthey J, Lauwers G, Esnaola N, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–36. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 30.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–15. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 31.Villa E, Colantoni A, Camma C, Grottola A, Buttafoco P, Gelmini R, et al. Estrogen receptor classification for hepatocellular carcinoma: comparison with clinical staging systems. J Clin Oncol. 2003;21:441–6. doi: 10.1200/JCO.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 32.Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, et al. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124–31. doi: 10.1016/j.jhep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Rabe C, Lenz M, Schmitz V, Pilz T, Fimmers R, Sauerbruch T, et al. An independent evaluation of modern prognostic scores in a central European cohort of 120 patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15:1305–15. doi: 10.1097/00042737-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Giannini E, Risso D, Botta F, Romagnoli P, Malfatti F, Fumagalli A, et al. Prognosis of hepatocellular carcinoma in anti-HCV positive cirrhotic patients: a single-centre comparison amongst four different staging systems. J Intern Med. 2004;255:399–408. doi: 10.1046/j.1365-2796.2003.01284.x. [DOI] [PubMed] [Google Scholar]
- 35.Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Hepatology. 2001;34:529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 36.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new staging system. Cancer. 2000;89:2266–73. [PubMed] [Google Scholar]
- 37.Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–5. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–19. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 39.Izumi R, Shimizu K, Li T, Yagi M, Matsui O, Nonomura A, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–7. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 40.Llovet JM, Bruix J, Fuster J, Castells A, García-Valdecasas JC, Grande L, et al. Liver transplantation for treatment of small hepatocellular carcinoma: the TNM classification does not have prognostic power. Hepatology. 1998;27:1572–7. doi: 10.1002/hep.510270616. [DOI] [PubMed] [Google Scholar]
- 41.Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer, 4th edn. Tokyo: Kanehara, 2000:19.[in Japanese]. [Google Scholar]
- 42.Henderson JM, Sherman M, Tavill A, et al. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB. 2003;5:243–50. doi: 10.1080/13651820310015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruix J, Llovet JM. Prognostic prediction on HCC: did anybody expect it to be easy? Hepatology. 2004;39:551–2. [Google Scholar]
- 44.Colombo M, Sangiovanni A. The strategic role of staging in the treatment of HCC. Hepatology. 2004;39:552–3. [Google Scholar]
- 45.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 46.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 47.Van de Vijver MJ, He YD, Van't Veer LJ. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]