Abstract
The majority of patients with pancreatic cancer present with disease that is unresectable due to local invasion. This article reviews the evidence (or lack thereof) that chemoradiation therapy (CRT) helps these patients in four areas: survival, tumor downstaging, palliation of obstructive symptoms, and pain control. We believe that CRT allows a small percentage of patients with locally advanced disease to undergo potentially curative resection while providing effective palliative treatment.
Keywords: Chemoradiation, neoadjuvant, pancreatic cancer, pancreaticoduodenectomy, palliation
Introduction
By the time patients present with pancreatic cancer, fewer than 10% have tumors that are amenable to potentially curative resection. Approximately one-third have distant metastatic disease and have a median survival of less than 6 months. The rest (over half of all patients with pancreatic cancer) have disease that is considered unresectable due to local invasion of adjacent structures. This heterogeneous group of patients can be challenging to treat, as they generally have problems related to their local tumor burden prior to developing distant metastatic disease.
The term ‘unresectable’ is somewhat subjective, as there are varying degrees of unresectability and varying opinions on what constitutes unresectable disease. We advocate neoadjuvant chemoradiation (CRT) for patients with both potentially resectable and locally advanced pancreatic cancer at our institution. We, however, attempt to make a distinction between truly unresectable disease and locally advanced disease.
Circumferential involvement or encasement of the superior mesenteric artery (SMA) or celiac axis represents truly unresectable disease. Although arterial resection and reconstruction has been described for this disease, the results are generally poor 1, due not only to the morbidity of the procedure but also to the perineural invasion often associated with arterial involvement 2. We do not necessarily classify non-circumferential arterial involvement or abutment as truly unresectable, as negative resection margins can sometimes be achieved after neoadjuvant CRT.
Although thrombosis of the superior mesenteric vein (SMV) or portal vein is associated with a prognosis similar to patients with distant metastatic disease, disease that involves the SMV and/or portal vein without thrombosis can be resected and reconstructed with good results 3,4,5,6. In contrast to arterial involvement, venous involvement does not necessarily portend a poorer prognosis when compared to tumors of similar size without venous involvement 3. Similarly, adjacent organ invasion—although associated with tumors of large size—is not technically unresectable.
Finally, regional lymph node disease is often discussed in the context of locally advanced disease, despite its different biology. Regional lymph node disease is often technically resectable: the question is whether or not it is appropriate to do so. Although it is clear that positive lymph nodes are one of the most important predictors of recurrence following resection 7, selected patients with positive lymph nodes probably do better with resection than without it. Regional lymph node disease should probably be considered separately from both locally advanced and truly unresectable disease.
Studies of'locally advanced' and'unresectable' pancreatic cancer have used various definitions of these terms, and comparison between studies can be difficult. Despite this limitation, a number of studies have established an important role for chemoradiation therapy (CRT) in the management of locally advanced or unresectable pancreatic cancer. This article reviews the evidence (or lack thereof) that CRT helps in four areas: survival, tumor downstaging, palliation of obstructive symptoms, and pain control.
Survival
A series of randomized controlled trials spanning the 1970s and 1980s provides strong evidence that the combination of chemotherapy and radiation therapy improves survival over either modality alone in patients with locally unresectable disease. In the 1969 landmark study by Moertel etal., survival was increased from 6 months with radiation alone to 10 months with 5FU plus XRT 8. A subsequent GI Tumor Study Group (GITSG) study demonstrated the superiority of 5FU plus XRT over XRT alone and even suggested a nonsignificant survival advantage for using 60 Gy over 40 Gy 9. Other randomized studies have corroborated the outcomes achieved with 5FU plus XRT but have not managed to significantly improve upon them by adding to or changing the chemotherapeutic regimen 10,11. Interestingly, no randomized controlled study had been performed comparing CRT to no CRT until a recent Japanese study demonstrated a median survival of 13 months in patients receiving XRT plus continuous infusion 5FU, compared to 6 months in patients receiving no treatment 12. These studies have all focused primarily on recurrence and survival as their endpoints, although no apparent difference was seen with respect to local versus distant disease progression.
Tumor downstaging
With the increased use of CRT for unresectable disease came the observations that occasional patients had dramatic responses to treatment that rendered them resectable. Numerous institutions have now reported their experiences with preoperative or'neoadjuvant' CRT. Patient selection criteria and CRT regimens have varied widely, and rates of resection following neoadjuvant CRT have varied from 0% to 60%, with most falling between 10% and 20% 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27.
At our institution, we have now neoadjuvantly treated almost 100 patients with'locally advanced' disease, which excludes patients with only venous abutment (potentially resectable) and excludes patients with venous thrombosis (truly unresectable). Over the years, our resectability rate has remained relatively constant at approximately 18% 25,28. Most of the patients who were successfully resected with negative margins had tumors with either venous involvement alone or with very limited arterial involvement (abutment). Interestingly, the median survival in this group of patients is 20 months 25, which is similar to patients at our institution who undergo surgery first, followed by adjuvant CRT, and better than patients treated with CRT alone.
To what extent this represents tumor downstaging is difficult to definitively determine without exploring all patients before and after CRT. In some cases, tumor abutment on CT may represent peritumor pancreatitis that resolves during CRT. Furthermore, a certain proportion of these patients might have been resectable if explored upfront, although the positive predictive value of unresectability by CT is felt to be greater than 90% 29,30. In general, radiographic responses to CRT have been modest 25,31,32. However, radiographic responses do not correlate well with histologic responses, and the replacement of tumor with fibrosis that has typically been observed on histologic examination of resected surgical specimens may result in little or no change in radiographic appearance. For instance, approximately 10% of tumors that appear locally advanced on restaging CT due to vascular involvement can be resected because only sterile fibrosis instead of viable tumor is found at exploration 33. However, when the complete tumor specimen is examined pathologically after resection, we have observed complete histologic responses in approximately 8% of patients with potentially resectable tumors treated with neoadjuvant CRT, but in none of the resected tumor specimens that were initially classified as locally advanced 34. It is our conclusion from these data that tumor downstaging does occur in a minority of patients with currently utilized regimens.
Palliation of obstructive symptoms
Although it seems intuitive that local tumor control should prevent or delay obstructive symptoms, very few studies specifically address this role of CRT. In the GITSG studies, for instance, an unspecified proportion of patients had previously undergone palliative surgical procedures. Most studies of palliative and neoadjuvant CRT have not appreciated high rates of isolated local progression, but local control is notoriously difficult to evaluate. To our knowledge, only one neoadjuvant CRT study has commented on their low rate (less than 10%) of GI complications in patients not undergoing resection 16. In our experience, median survival in patients with locally advanced tumors who do not undergo resection following neoadjuvant CRT is approximately 10 months. Gastrojejunostomy and/or biliary bypass had been performed prior to CRT in 8% of patients and following CRT in approximately 20% of patients at the time of exploration to determine resectability.Only an additional 8% of patients required gastrojejunostomy for symptoms of gastric outlet obstruction. Palliative surgical procedures were therefore performed in almost 40% of unresectable patients but were prophylactic in most. Prophylactic gastrojejunostomy has been recommended to prevent an estimated risk of gastric outlet obstruction of almost 20% 35, whereas a more selective approach to surgical palliation has been recommended by others 36. These studies did not specify what proportion of patients received palliative CRT. We believe that we may be over-treating by performing prophylactic surgical bypasses in patients receiving CRT. The combination of endoscopic stenting and CRT may provide adequate palliation and obviate the need for surgical bypasses in the majority of these patients.
Pain control
For most patients with incurable pancreatic cancer, pain control is or will become the most important goal for improving the quality of their remaining life. Increasing attention has been devoted to the incorporation of quality of life endpoints into clinical trials. A'clinical benefit score', assessing pain, weight gain, and performance status, was developed by Burris etal., for the evaluation of chemotherapy for metastatic pancreatic cancer 37. When applied to patients receiving CRT for locally advanced pancreatic cancer, 6 of 25 patients improved in at least one category without deteriorating in another 38. With respect to pain control specifically, 5 of 25 patients had a sustained decrease in analgesic consumption while only 2 patients had an increase in analgesic consumption. In the Japanese study mentioned above comparing XRT plus continuous infusion 5FU to no treatment, 8 of 10 patients with pain prior to treatment experienced pain relief lasting a median of 5 months 12.
Management of locally advanced pancreatic cancer
CRT clearly improves survival, probably provides pain control, and possibly helps to prevent obstructive symptoms in patients with locally advanced pancreatic cancer. CRT deserves to be considered the standard-of-care for the palliative treatment of these patients. In addition, we believe that there is compelling evidence that a minority of patients with locally advanced but not truly unresectable disease can be rendered resectable by neoadjuvant CRT. Depending on the institution and its available protocols, it may or may not be necessary to distinguish between CRT with neoadjuvant versus palliative intent, other than for providing patients with realistic expectations. Many radiation oncologists will treat palliatively with up to 60 Gy and utilize a more limited field. Our bias is to treat with neoadjuvant intent, unless the patient has truly unresectable disease, as defined above (arterial encasement or venous thrombosis).
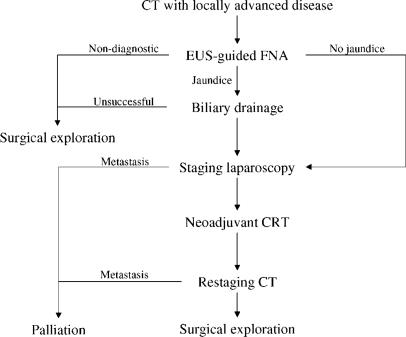
To summarize our treatment approach to patients with locally advanced pancreatic cancer, all patients first need a high-quality contrast-enhanced CT scan to assess for distant metastatic disease and local resectability. In jaundiced patients, ERCP is performed for common bile duct brushings and for endoscopic stent placement. In patients with truly unresectable disease, metallic Wallstents may be more durable than the typical plastic stents 39. If endoscopic biliary drainage is unsuccessful, percutaneous biliary drainage can usually be attained. If a cytologic diagnosis is not obtained by ERCP, a tissue diagnosis must be obtained by either image-guided percutaneous FNA or by EUS-guided FNA prior to initiation of neoadjuvant or palliative CRT. The presence of enlarged lymph nodes on CT is relatively non-specific, and we attempt to confirm regionally metastatic disease by EUS-guided FNA. The role of staging laparoscopy prior to CRT with neoadjuvant intent is unclear. One argument against laparoscopy in this setting is that patients with small-volume metastatic disease are probably not harmed and may even be benefited by CRT. However, for the purpose of studying the efficacy of neoadjuvant CRT, staging laparoscopy is helpful in excluding—to the extent possible—patients with metastatic disease. Following CRT, patients undergo restaging CT scan. The most important role of rest-aging CT is the identification of distant metastatic disease, which is consistently found in approximately 20% of patients following CRT. Our impression has been that the appearance of arterial abutment or even encasement on CT—typically considered indicative of unresectability—may represent sterile fibrosis. EUS with FNA has been employed in some patients to obtain cytopathologic evidence that viable tumor cells are present. Unless there is confirmation of arterial involvement or evidence of true unresectability by CT, we give patients'the benefit of the doubt' and offer exploration for possible resection. An overview of our current algorithm for locally advanced patients is shown in Figure 1.
Figure 1. .
An overview of our treatment approach to patients with locally advanced pancreatic cancer.
While the percentage of locally advanced patients able to undergo potentially curative resection is admittedly small with currently utilized CRT regimens, this approach provides patients with a small but realistic hope for longer survival without depriving them of effective palliative treatment.
References
- 1.Tamura K, Kin S, Ono K, Nagami H, Teramoto M, Tarumi T, Nakase A. [Operative results in cancer of the pancreas, especially complicated with large vascular involvement] Nippon Geka Gakkai Zasshi. 1989;90:1032–42. [PubMed] [Google Scholar]
- 2.Nagakawa T, Kayahara M, Ueno K, Ohta T, Konishi I, Ueda N, Miyazaki I. A clinicopathologic study on neural invasion in cancer of the pancreatic head. Cancer. 1992;69:930–5. doi: 10.1002/1097-0142(19920215)69:4<930::aid-cncr2820690416>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrman GM, Leach SD, Staley CA, Cusack JC, Charnsangavej C, Cleary KR, El-Naggar AK, Fenoglio CJ, Lee JE, Evans DB. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg. 1996;223:154–62. doi: 10.1097/00000658-199602000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launois B, Stasik C, Bardaxoglou E, Meunier B, Campion JP, Greco L, Sutherland F. Who benefits from portal vein resection during pancreaticoduodenectomy for pancreatic cancer? World J Surg. 1999;23:926–9. doi: 10.1007/s002689900601. [DOI] [PubMed] [Google Scholar]
- 5.Taschieri AM, Elli M, Rovati M, Sampietro GM, Cristaldi M, Danelli P, Pisacreta M. Surgical treatment of pancreatic tumors invading the spleno-mesenteric-portal vessels. An Italian Multicenter Survey. Hepatogastroenterology. 1999;46:492–7. [PubMed] [Google Scholar]
- 6.van Geenen RC, ten Kate FJ, de Wit LT, van Gulik TM, Obertop H, Gouma DJ. Segmental resection and wedge excision of the portal or superior mesenteric vein during pancreatoduodenectomy. Surgery. 2001;129:158–63. doi: 10.1067/msy.2001.110221. [DOI] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 8.Moertel CG, Childs DS, Jr, Reitemeier RJ, Colby MY, Jr, Holbrook MA. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–7. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 9.Moertel C, Frytak S, Hahn R, O'Connell M, Reitemeier R, Rubin J, Schutt A, Weiland L, Childs D, Holbrook M, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads) + 5-fluorouracil, and high-dose radiation + 5-fluorouracil. The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–10. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.[Anonymous], Radiation therapy combined with Adriamycin or 5-fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Gastrointestinal Tumor Study Group. Cancer 1985;56:2563–8. [DOI] [PubMed] [Google Scholar]
- 11.[Anonymous], Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 1988;80:751–5. [PubMed] [Google Scholar]
- 12.Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, Aikou T. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146–50. doi: 10.1016/s0360-3016(01)02806-1. [DOI] [PubMed] [Google Scholar]
- 13.Jeekel J, Treurniet-Donker AD. Treatment perspectives in locally advanced unresectable pancreatic cancer. Br J Surg. 1991;78:1332–4. doi: 10.1002/bjs.1800781121. [DOI] [PubMed] [Google Scholar]
- 14.Jessup JM, Steele G, Jr, Mayer RJ, Posner M, Busse P, Cady B, Stone M, Jenkins R, Osteen R. Neoadjuvant therapy for unresectable pancreatic adenocarcinoma. Arch Surg. 1993;128:559–64. doi: 10.1001/archsurg.1993.01420170093014. [DOI] [PubMed] [Google Scholar]
- 15.Coia L, Hoffman J, Scher R, Weese J, Solin L, Weiner L, Eisenberg B, Paul A, Hanks G. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys. 1994;30:161–7. doi: 10.1016/0360-3016(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 16.Kamthan AG, Morris JC, Dalton J, Mandeli JP, Chesser MR, Leben D, Cooperman A, Bruckner HW. Combined modality therapy for stage II and stage III pancreatic carcinoma. J Clin Oncol. 1997;15:2920–7. doi: 10.1200/JCO.1997.15.8.2920. [DOI] [PubMed] [Google Scholar]
- 17.Safran H, Akerman P, Cioffi W, Gaissert H, Joseph P, King T, Hesketh PJ, Wanebo H. Paclitaxel and concurrent radiation therapy for locally advanced adenocarcinomas of the pancreas, stomach, and gastroesophageal junction. Semin Radiat Oncol. 1999;9:53–7. [PubMed] [Google Scholar]
- 18.Bajetta E, Di Bartolomeo M, Stani SC, Artale S, Ricci SB, Bozzetti F, Mazzaferro V, Toffolatti L, Buzzoni R. Chemo-radiotherapy as preoperative treatment in locally advanced unresectable pancreatic cancer patients: results of a feasibility study. Int J Radiat Oncol Biol Phys. 1999;45:285–9. doi: 10.1016/s0360-3016(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 19.Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, Savage PD, Tepper JE. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–12. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 20.Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89:314–27. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Kornek GV, Schratter-Sehn A, Marczell A, Depisch D, Karner J, Krauss G, Haider K, Kwasny W, Locker G, Scheithauer W. Treatment of unresectable, locally advanced pancreatic adenocarcinoma with combined radiochemotherapy with 5-fluorouracil, leucovorin and cisplatin. Br J Cancer. 2000;82:98–103. doi: 10.1054/bjoc.1999.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanebo HJ, Glicksman AS, Vezeridis MP, Clark J, Tibbetts L, Koness RJ, Levy A. Preoperative chemotherapy, radiotherapy, and surgical resection of locally advanced pancreatic cancer. Arch Surg. 2000;135:81–7. doi: 10.1001/archsurg.135.1.81. [DOI] [PubMed] [Google Scholar]
- 23.Kastl S, Brunner T, Herrmann O, Riepl M, Fietkau R, Grabenbauer G, Sauer R, Hohenberger W, Klein P. Neoadjuvant radio-chemotherapy in advanced primarilynon-resectable carcinomas of the pancreas. Eur J Surg Oncol. 2000;26:578–82. doi: 10.1053/ejso.2000.0950. [DOI] [PubMed] [Google Scholar]
- 24.Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, Niederhuber J, Bastidas JA. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2001;5:27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 25.White R, Hurwitz H, Lee C, Anscher M, Paulson E, Gottfried M, Baillie J, Branch M, Jowell P, McGrath K, Clary B, Pappas T, Tyler D. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol. 2001;8:758–65. doi: 10.1007/s10434-001-0758-1. [DOI] [PubMed] [Google Scholar]
- 26.Crane CH, Abbruzzese JL, Evans DB, Wolff RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters PW, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002;6:763–9. doi: 10.1016/s1091-255x(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 28.White R, Lee C, Anscher M, Gottfried M, Wolff R, Keogan M, Pappas T, Hurwitz H, Tyler D. Preoperative chemoradiaion for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol. 1999;6:38–45. doi: 10.1007/s10434-999-0038-z. [DOI] [PubMed] [Google Scholar]
- 29.Fuhrman G, Charnsangavej C, Abbruzzese J, Cleary K, Martin R, Fenoglio C, Evans D. Thin-section contrast-enhanced computed tomography accurately predicts the resectabihty of malignant pancreatic neoplasms. Am J Surg. 1994;167:104–13. doi: 10.1016/0002-9610(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 30.Loyer EM, David CL, Dubrow RA, Evans DB, Charnsangavej C. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom Imaging. 1996;21:202–06. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 31.Spitz F, Abruzzese J, Lee J, Pisters P, Lowy A, Fenoglio C, Cleary K, Janjan N, Goswitz M, Rich T, Evans D. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928–37. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman JP, Lipsitz S, Pisansky T, Weese JL, Solin L, Benson AB., 3rd Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317–23. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 33.White RR, Paulson EK, Freed K, Keogan M, Hurwitz H, Lee C, Morse MA, Gottfried MR, Baillie J, Branch MS, et al. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J Gastrointest Surg. 2001;5:626–33. doi: 10.1016/s1091-255x(01)80105-0. [DOI] [PubMed] [Google Scholar]
- 34.White R, Xie H, Gottfried M, Hurwitz H, Morse M, Czito B, Paulson E, Clary B, Pappas T, Tyler D. Prognostic significance of histologic response to preoperative chemoradiation for pancreatic cancer(submitted). [Google Scholar]
- 35.Lillemoe KD, Cameron JL, Hardacre JM, Sohn TA, Sauter PK, Coleman J, Pitt HA, Yeo CJ. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322–8. doi: 10.1097/00000658-199909000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espat N, Brennan M, Conlon K. Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg. 1999;188:649–57. doi: 10.1016/s1072-7515(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 37.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 38.Fisher BJ, Perera FE, Kocha W, Tomiak A, Taylor M, Vincent M, Bauman GS. Analysis of the clinical benefit of 5-fluorouracil and radiation treatment in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1999;45:291–5. doi: 10.1016/s0360-3016(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Hirai R, Kitagawa M, Takehira Y, Yamada M, Tamakoshi K, Kobayashi Y, Nakamura H, Kanamori M. Treatment of common bile duct obstruction by pancreatic cancer using various stents: single-center experience. Cardio-vasc Intervent Radiol. 2002;25:373–80. doi: 10.1007/s00270-002-0426-2. [DOI] [PubMed] [Google Scholar]