Abstract
Hypothesis. Frozen section diagnosis and permanent diagnosis of bile duct margin predict local recurrence after surgical resection of gallbladder or bile duct carcinoma. Design. Retrospective review. Setting. University, tertiary care. Patients. A total of 20 patients underwent frozen section diagnosis of bile duct margin for resection of gallbladder and bile duct carcinoma. Main outcome. Diagnosis of frozen and permanent section of bile duct margin, and local recurrence. Results. The permanent diagnosis was identical in 15 patients but changed in 5 (from positive to negative in 3 and from negative to positive in 2). The reasons for these changes were overdiagnosis (mucosal lesions in two and mesenchymal components in another) and new recognition of malignant cells on permanent section in the other two. In seven patients with a positive bile duct margin by permanent histology, mucosal spread was evident in two and involvement of the subepithelial layer was present in the other five. No local recurrence occurred in the two patients with epithelial spread and four of the five with subepithelial infiltration. Conclusions. Frozen section and permanent diagnoses of the bile duct margin in gallbladder and bile duct carcinoma may be inconsistent in 25% of patients due to overdiagnosis of frozen section or new recognition of cancer cells by permanent histology. In situ carcinoma does not always produce local recurrence, while cancer cells in the subepithelial layer strongly predict occurrence of local recurrence.
Keywords: Bile duct cancer, bile duct margin, frozen section diagnosis
Introduction
The clinical course of patients with gallbladder or bile duct carcinoma remains dismal despite the recent advances of diagnostic and therapeutic modalities 1,2,3. Intramural and/or periductal invasion determined microscopically is sometimes more extensive than the invasion evaluated preoperatively. Some report a strong correlation between positive surgical margins and postoperative local recurrence 4,5,6. Frozen sections are usually performed to decide the cutting margins of resection of the bile duct. However, the disagreement between frozen section diagnosis and permanent paraffin section diagnosis has been reported 7,8 and the surgeons could be embarrassed by any alteration of diagnosis 7. An accurate assessment of frozen section is mandatory to decide the optimal operative procedure. There are many surgical margins including the bile duct in gallbladder or bile duct carcinoma, and recurrent modes including local recurrence and distant metastasis. In this communication, we concentrate on frozen section and permanent diagnoses of the bile duct margins and review their potential surgical implications on local recurrences.
Materials and methods
This series is composed of 44 patients with gallbladder or bile duct cancer who underwent surgical resection at the Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, from January 1995 to December 2001, and whose follow-up imagings or autopsy findings were available more than one year after operation. The 44 tumors were Tl in four, T2 in six, T3 in 31 and T4 in the other three by TNM classification 9. In 20 of the 44 patients, frozen section diagnosis of the resected bile duct margin was done during the operation. These 20 tumors were Tl in 2, T2 in 3, T3 in 13 and T4 in the remaining 2. For frozen section, the bile duct margin tissue was mounted in optimum cutting temperature (OCT) compound (Miles Inc. Elkhart, IN, USA), frozen in liquid nitrogen and thin sections were cut from the OCT compound blocks using a cryostat. The sections were stained by hematoxylin and eosin and subjected to microscopic diagnosis. After frozen section diagnosis, the tissues were fixed in formalin and embedded in paraffin. Thin sections were cut from paraffin-embedded blocks with a microtome and were stained with hematoxylin and eosin. Permanent diagnosis was made under microscopy. Frozen section and permanent diagnoses were compared with each other.
The presence or absence of local recurrence more than one year after surgery was determined by autopsy in six patients or by imagings including ultrasonography, computed tomography and magnetic resonance imagings in the other 39 (Figures 1 and 2). The criteria of the local recurrence were defined as recurrent mass within the resection field. In this series, ductal margin was examined and radial margins were not analyzed. The distribution of the patients was examined by the chi-squared test.
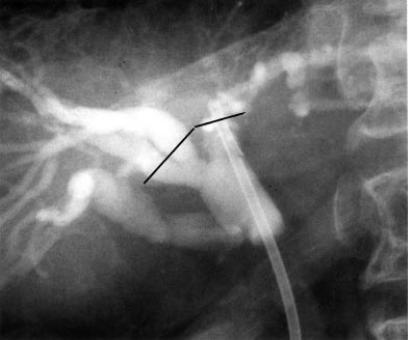
Figure 1. .
Percutaneous cholangiography shows irregular stenosis of the hilar bile duct. Straight lines indicate cutting lines performed.
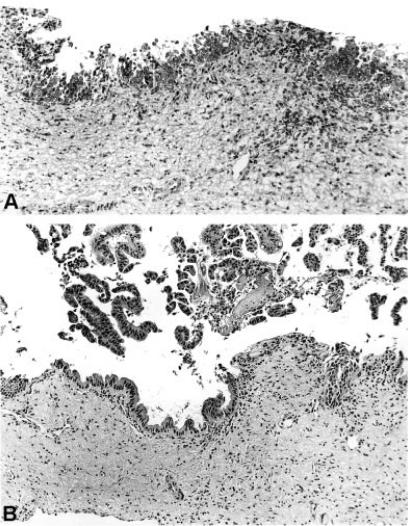
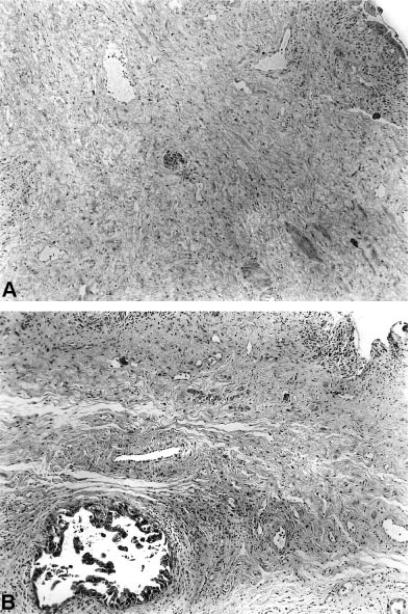
Figure 2. .
Frozen section histology (straight lines of Figure 1) shows atypical epithelial cells mimicking in situ carcinoma (A) and permanent tissue section shows dysplastic epithelium of moderate degree (B) (H & E×l26).
Results
Frozen section and permanent diagnoses
Cancer cells were present at the bile duct margin in 3 of the 20 patients by frozen section histology and absent in the other 17. The diagnosis was altered from frozen to permanent section in 5 (25%) of the 20 patients: from positive to negative in 3 (Figures 123) and from negative to positive in the other 2 (Figures 4 and 5), while the diagnosis did not change in the other 15 (75%) (Table I).
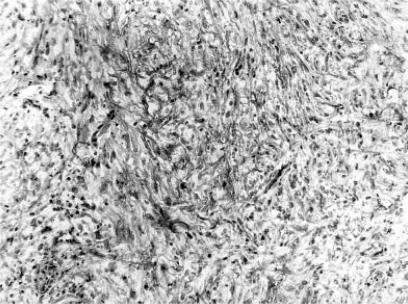
Figure 3. .
Frozen section histology shows immature mesenchymal cells probably induced by the placement of the PTBD tube (H & E×126).
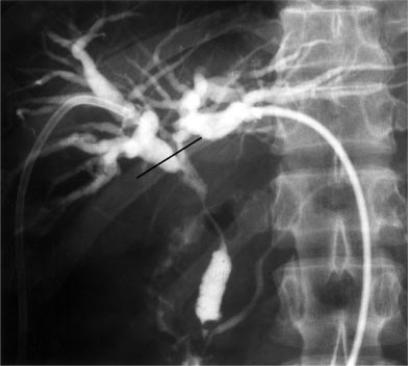
Figure 4. .
Percutaneous cholangiography shows irregular stenosis of the bile duct. Straight line shows the resectional margin.
Figure 5. .
Frozen section histology (straight line of Figure 4) shows no cancer cells (A) and paraffin section shows cancer cells in the subepithelial layer (B) (H & E×86).
Table I. Frozen section diagnosis, permanent diagnosis of frozen section and local recurrence.
| Frozen section | Permanent diagnosis of frozen section | Local recurrence | ||
|---|---|---|---|---|
| Yes (3)* | → | No (3) | → | Yes (1), No (2) |
| No (2) | → | Yes (2) | → | No (2) |
| No (15) | → | No (15) | → | Yes (5), No (10) |
*Numbers in parentheses indicate the number of patients.
In the three patients with the positive to negative change of the diagnosis, no cancer cells were recognized in the permanent section but local recurrence occurred in one of the three patients. The reasons for the changes in the diagnosis were overdiagnosis of dysplastic cells in the epithelial layer in two (Figure 2) and immature mesenchymal cells as malignant epithelium showing sarcomatous changes in another (Figure 3). This might have been produced by mucosal irritation caused by the of percutaneous transhepatic biliary drainage (PTBD) tube.
In the two patients with the negative to positive change of the diagnosis, cancer cells were absent on frozen section but appeared on permanent section (Figures 4 and 5). For the permanent diagnosis of frozen section histology, frozen tissues remaining after frozen histology were re-embedded in paraffin and cut for permanent diagnosis. Therefore, the cut-surface of the permanent histology was different from that of frozen section histology. No local recurrence was recognized in the two patients.
Of the 15 patients whose bile duct margins were negative both on frozen section and permanent section, local regional recurrence happened in 5 patients but not in the other 10.
Permanent diagnosis of bile duct and local recurrence
By paraffin section histology, the bile duct margin was affected by malignant cells in 7 patients but not in the other 37 (Table II). Local recurrence occurred in 4 (57%) of the 7 margin-positive patients, and in 9 (24%) of the 37 margin-negative patients (p = 0.081). Local recurrence was not related to T-stage.
Table II. Relationship between positive bile duct margin and local recurrence.
| Local recurrence | ||
|---|---|---|
| Positive bile duct margin | Yes | No |
| No | 9 | 28 |
| Yes | 4 | 3 |
p=0.081.
When local recurrence was examined by the layer of the positive bile duct margin, none (0%) of the two patients with cancer cells in the epithelial layer had local recurrence, while four (80%) of the five patients with cancer cells in the subepithelial layer developed regional recurrence (p = 0.053) (Table III).
Table III. Relationship between the layer of the positive bile duct margin and local recurrence.
| Local recurrence | ||
|---|---|---|
| Layer of positive bile duct margin | Yes | No |
| Epithelial layer | 0 | 2 |
| Subepithelial layer | 4 | 1 |
p=0.053.
Discussion
Frozen section histology is usually used to confirm cancer negative margins 10. However, frozen section histological results are sometimes divergent from permanent sections 7,8. To identify the surgical implications of frozen section and permanent diagnoses of bile duct resectional margins in gallbladder or bile duct carcinoma, our experiences were reviewed retrospectively. Of 20 patients who underwent frozen section diagnosis of the bile duct, the diagnosis was changed in 5 patients. In seven patients with a positive bile duct margin by permanent histology, mucosal spread was evident in two and involvement of the subepithelial layer was present in the other five. No local recurrence occurred in the two patients with a positive epithelial margin but regional recurrence occurred in four of the five patients with a positive subepithelial margin. These findings suggest that frozen section histology of the bile duct margin should be considered separately by dividing into epithelial and subepithelial lesions. In situ carcinoma does not always produce local recurrence, while cancer cells in the subepithelial layer strongly predict occurrence of local recurrence.
Some report a strong correlation between positive surgical margins and postoperative local recurrence 4,5,6, while others report no correlation between positive surgical margins and local recurrence 11. There are many surgical margins in gallbladder or bile duct carcinoma and, in this communication, the relationship between the bile duct margin and local recurrence was examined. Local recurrence occurred in 4 (57%) of the 7 positive patients and in 9 (24%) of the 37 negative patients, showing a marginally significant relationship (p=0.081).
Usually permanent section diagnosis is made based on structural and cytological dysplasia, while frozen section diagnosis mainly relies on structural atypia 10. Frozen section diagnosis is often difficult even for experienced pathologists and cytologists. Bile duct margin diagnosis should be considered by distinguishing in situ epithelial and subepithelial lesions. Diagnosis of mucosal infiltration of malignant cells is difficult even on permanent sections and is very difficult on frozen sections. Moreover, the diagnosis of the grade of dysplasia is different from pathologist to pathologist. The fate of such dysplastic epithelium remains unclear. In the two patients with in situ carcinoma in this series, no local recurrence was observed.
Diagnosis of invasive carcinoma in the subepithelial layer is easy especially when there is perineural invasion. Invasive carcinoma in the subepithelial layer of resected bile duct margins can be considered to have a high potential of local recurrence 12. In the present series, four of the five patients with positive margins in the subepithelial layer developed local recurrence. Immature mesenchymal cells induced by the placement of PTBD tube may be difficult to differentiate from sarcomatous carcinoma, as shown in one patient in this series. In this situation, cytokeratin immunostaining may be useful to differentiate epithelial cells from mesenchymal cells. Mucous glands in the subepithelial layer should be also distinguished from malignant tubules infiltrating into the subepithelial layer while preserving the forms of tubules.
At the operating theater, direct communication between the surgeons and pathologists are mandatory because detailed information as to the diagnosis is difficult to convey through indirect transportation by residents or technicians. Direct communication about the meanings of the frozen section diagnosis is mandatory, including the site of dysplastic cells in the bile duct margin and grade of dysplasia. Surgeons should finally decide the optimal cutting sites during the operation based on the preoperative imagings, including direct cholangiography, anatomical limitation of resection, limits of frozen section diagnosis, patients' condition and informed consent of the patients and his/her family.
Regarding frozen section diagnosis of the bile duct margin in gallbladder or bile duct carcinoma, in situ dysplastic lesions should be considered carefully, while carcinoma cells in the subepithelial layer should be taken into account seriously.
References
- 1.Yamaguchi K, Enjoji M, Nakayama F. Cancer of extrahepatic bile duct: A clinicopathologic study of immunohisto-chemistry for CEA, CA19-9 and p21. World J Surg. 1988;12:11–17. doi: 10.1007/BF01658480. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi K, Chijiiwa K, Saiki S, Shimizu S, Takashima M, Tanaka M. Carcinoma of the extrahepatic bile duct: Mode of spread and its prognostic significance. Hepato-Gastroenterology. 1997;44:1256–61. [PubMed] [Google Scholar]
- 3.Yamaguchi K, Chijiiwa K, Saiki K, Nishihara K, Takashima M, Kawakami K, Tanaka M. Retrospective analysis of 70 operation for gallbladder carcinoma. Br J Surg. 1997;84:200–4. [PubMed] [Google Scholar]
- 4.Bengmark S, Ekberg H, Evander A, Klofver-Stahl B, Tranberg KG. Major liver resection for hilar cholangiocarcinoma. Ann Surg. 1988;207:120–5. doi: 10.1097/00000658-198802000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beazley R, Hadjis N, Benjamin I, Blumgart L. Clinico-pathological aspects of hilar bile duct cancer. Experience with resection and bypass surgical treatments. Ann Surg. 1984;199:623–36. doi: 10.1097/00000658-198406000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–8. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strong R, Lynch S. Surgical resection for hilar cholangiocarcinoma. J Hep Bil Pancreat Surg. 1995;2:233–8. [Google Scholar]
- 8.Okazaki Y, Horimi T, Kotaka M, Morita S, Takasaki M. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepato-Gastroenterology. 2002;49:625–7. [PubMed] [Google Scholar]
- 9.Sobin LH, Witterkind C, International Union against Cancer (UICC). TNM Classification of malignant tumours. 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 10.Yamaguchi K, Chijiiwa K, Saiki K, Shimizu S, Tsuneyoshi M, Tanaka M. Reliability of frozen section diagnosis of gallbladder tumor for detecting carcinoma and depth of its invasion. J Surg Oncol. 1997;65:132–6. doi: 10.1002/(sici)1096-9098(199706)65:2<132::aid-jso11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Bhuiya M, Nimura Y, Kamiya J, Kondo S, Nagino M, Hayakawa N. Clinicopathologic factors influencing survival of patients with hilar bile duct carcinoma: Multivariate statistical analysis. World J Surg. 1993;17:653–7. doi: 10.1007/BF01659134. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg. 1992;163:382–6. doi: 10.1016/0002-9610(92)90038-s. [DOI] [PubMed] [Google Scholar]