Abstract
Background. Obesity is a risk factor for cholesterol gallstone formation, but the pathogenesis of this phenomenon remains unclear. Most human obesity is associated with diabetes and leptin-resistance. Previous studies from this laboratory have demonstrated that diabetic leptin-resistant (Lepdb) obese mice have low biliary cholesterol saturation indices, enlarged gallbladders and diminished gallbladder response to neurotransmitters. Recently, a novel leptin-resistant mouse strain Leprdb-rtnd (Rotund) has been discovered. Rotund mice are also obese, diabetic, and have an abnormal leptin receptor. Therefore, we tested the hypothesis that leptin-resistant obese Rotund mice would have large gallbladders and reduced biliary motility.
Methods. Eight-week-old control (C57BL/6J, N=12) and Rotund leptin-resistant (Leprdb-rnd, N=9) mice were fed a non- lithogenic diet for four weeks. Animals were fasted and underwent cholecystectomy. Gallbladder volumes were recorded, and contractile responses (N/cm2) to acetylcholine (10−5 M), Neuropeptide Y (10−8,−7,−6 M), and cholecystokinin (10−10,−9,−8,−7 M) were measured. Results were analyzed using the Mann-Whitney Rank Sum Test.
Results. Compared to control mice, Rotund mice had larger body weights, higher serum glucose levels, and greater gallbladder volumes (p<0.05). Rotund gallbladders had less contractility (p<0.05)) to acetylcholine and cholecystokinin than control mice. Responses to Neuropeptide Y were also less, but not statistically significant, in the Rotund mice.
Conclusions. These data suggest that leptin-resistant Rotund mice have (1) enlarged gallbladders with (2) diminished contractility compared to lean control mice. Therefore, this study confirms that leptin-resistance is associated with abnormal biliary motility and may lead to gallstone formation in leptin-resistant obesity.
Keywords: Cholesterol, diabetes mellitus, gallbladder, gallstones, motility
Introduction
Obesity has reached epidemic proportions in the United States and many westernized countries. More than 50 million US adults have a body mass index (BMI) greater than 30 1. Human obesity is associated with insulin-resistant diabetes mellitus, elevated serum lipids, and a 3.7 times greater risk of gallstone disease 2. The majority of obese humans are resistant to leptin, a hormone produced by adipocytes that induces satiety and regulates energy expenditure. While short forms of the leptin receptor are known to exist, leptin is thought to work primarily on the long form receptor, which is highly prevalent in the hypothalamus but is also found throughout the gastrointestinal tract 3. However, the exact relationship among obesity, leptin, and cholesterol gallstone disease is still unknown. In contrast, gallstone formation is known to require the interplay of three factors, cholesterol supersaturation of bile, cholesterol crystal pronucleators, and biliary stasis.
The long form of the leptin receptor is highly conserved between mice and humans and is defective in the leptin-resistant (Lepdb) mouse model 4. Previous studies from our laboratory have shown that when leptin-resistant obese mice (Lepdb) are fed a standard, low cholesterol diet, they have enlarged gallbladders with diminished contraction to neuro-transmitter stimulation 5. Lepdb mice have an absent signal transcription and translation (STAT) region of the leptin receptor, but have a normal extracellular domain and leptin-binding capacity as well as a normal janus kinase (JAK) region 6. Because JAK can phosphorylate and activate other pathways besides STAT 7, the Lepdb mouse model is not a true null leptin receptor model. Recently, a new leptin-resistant Rotund mouse has been characterized 6. This mouse has a nucleotide deletion resulting in a premature stop codon and a severely truncated leptin receptor, which is devoid of all extracellular and intracellular domains. Therefore, using a true leptin receptor deficient model, we hypothesize that the Rotund mouse will also demonstrate elevated glucose levels, obesity, enlarged gallbladder volumes, and decreased gallbladder motility when compared to control mice.
Materials and methods
Animals and diets
To study gallbladder contraction, 12 lean control C57BL/6J and 9 Rotund 7-week-old female mice were obtained by special permission from a laboratory affiliated with Jackson Laboratory (Bar Harbor, ME). The mice were housed in cages up to 5 mice each in a light (6 am–6 pm) and temperature (22°C) controlled environment, and isolation precautions were observed. All mice received a standard low cholesterol CHOW diet (Ralston Purina, St. Louis, MO) for 4 weeks. At 12 weeks of age, all mice were fasted overnight. Upon study, mice were anesthetized with xylazine (15 mg/kg, Phoenix Pharmaceuticals, Burnsville, MN) and keta-mine (50 mg/kg, Phoenix Pharmaceuticals, Burnsville, MN), weighed, and underwent cholecystectomy. Gallbladders were placed in ice cold, preoxygenated modified Krebs solution (in mmol/L: NaCl, 116.6; NaCO3, 21.9; KH2PO4, 1.2; glucose, 5.4; MgCl2, 1.2; KCl, 3.4; and CaCl2 2.5). Whole blood was obtained by aspiration from the right heart, and livers were removed and weighed.
Bile and Serum Glucose Analysis
Bile was aspirated from the fundus of intact gallbladders with a 30-gauge needle, placed into a micro-tube, centrifuged at 15,000 rpm for 5 min at room temperature (Micromax model, International Equipment Company, Needham Heights, MA), and measured with a micropipette. Whole blood was also centrifuged at 15,000 rpm for 5 min at room temperature to separate serum. Serum was warmed to 39°C, and glucose was measured with Freestyle glucose strips and glucometer (Therasense, Alameda, CA).
In-vitro muscle bath
Gallbladders were sutured with 7-0 polypropylene sutures at both ends and suspended longitudinally in 3 mL muscle bath wells filled with modified Krebs solution, warmed to 39°C, and oxygenated with 95% O2 and 5% CO2. Gallbladders were equilibrated at 0.025 grams of tension. Optimal length was then determined by stimulation with 10−5 M acetycholine (ACh, Sigma Chemical, St. Louis, MO) at 0.025 gram increments until maximal gallbladder contraction was obtained. Gallbladders were maintained at their optimal lengths while Neuropeptide Y (NPY, Sigmal Chemical) at 10−8, 10−7, and 10−6 M doses and cholecystokinin octapeptide (CCK, Sigmal Chemical) at 10−10, 10−9, 10−8, and 10−7 M doses were added. Responses were measured with the Windaq/Ex computer software (Dataq Instruments, Inc., Akron, Ohio). After every neurotransmitter dosing and after every 15 minutes, gallbladders were rinsed with modified Krebs solution. Gallbladder lengths and weights were measured and used to calculate the cross-sectional area. Gallbladder contractile responses were normalized for area and were expressed as Newtons per centimeter squared (N/cm2).
Statistical analysis
Data analyses were performed with SigmaStat Statistical Software (Jandel Corporation, San Rafael, CA). All data are expressed as mean±SEM. Mouse body and liver weights, serum glucose, gallbladder volume, and neurotransmitter responses were analyzed by the Mann-Whitney Rank Sum Test. A p-value less than 0.05 was regarded as significant.
Results
Body and liver weights, serum glucose, and gallbladder volume
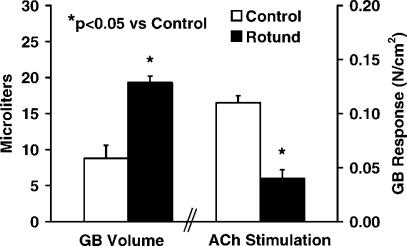
Data for body and liver weights, serum glucose levels and gallbladder volumes are shown in Table I and Figure 1. The body and liver weights of the Rotund mice were dramatically larger than the control animals (p<0.001). In addition, the serum glucose levels of the Rotund mice were markedly greater than the glucose levels of the C57 control mice (421 and 160 µmg/dL, respectively, p<0.01). The gallbladder volumes (Figure 1) of the Rotund mice were 19.3 µmL, which were significantly larger than 8.8 µmL average gallbladder volume of the control mice (p<0.05).
Table I. Body weight, liver weight, and serum glucose levels for control and rotund mice.
| Strain | Body weight | Liver weight | Glucose |
|---|---|---|---|
| Control | 17.2±0.4 | 0.87±0.05 | 160±23 |
| Rotund | 40.0±2.0* | 2.16±0.14* | 421±37* |
Values are mean±SEM, body and liver weights are shown in grams, and serum glucose levels are given as mg/dL.
*p<0.01 versus Control.
Figure 1. .
Gallbladder volume and response to acetylcholine stimulation.
Muscle bath
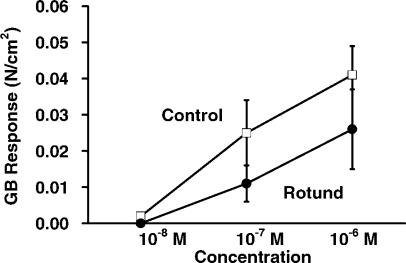
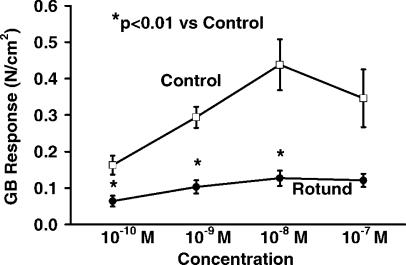
Gallbladder responses to ACh are also shown in Figure 1. The contractile responses of leptin-resistant Rotund mice were significantly less than the responses of the control mice (0.04 versus 0.11 N/cm2, p<0.05). Gallbladder responses to NPY at the 10−8, 10−7, and 10−6 M concentrations are shown in Figure 2. Again, the control mice had the higher contractility than the Rotund mice, but these differences were not statistically significant. The gallbladder responses to CCK at the 10−10,10−9, 10−8 M concentrations (Figure 3) were also significantly higher (p<0.01) in the control mice compared to the Rotund mice.
Figure 2. .
Gallbladder response to neuropeptide Y.
Figure 3. .
Gallbladder response to cholecystokinin.
Discussion
In this study, Rotund leptin-resistant mice fed a standard low cholesterol diet for four weeks demonstrated heavier body and liver weights and dramatically elevated serum glucose levels compared to lean C57 control mice. In addition, the Rotund mice had enlarged gallbladders at rest, along with reduced contractility to neurotransmitters in an in-vitro muscle bath. These results are consistent with prior studies from our laboratory with the leptin-resistant Lepdb mouse, which also has high serum glucose levels, enlarged gallbladders, and diminished motility to ACh, NPY, and CCK 5. These similarities are not surprising, as both of these mice have defective leptin receptors and similar, but not identical, phenotypes.
The defect in motility demonstrated in both of the leptin-resistant obese mice is also similar to our findings with Lepob mice, which lack the ability to produce leptin and, therefore, are leptin-deficient. Lepob mice also have severe insulin resistant diabetes, hyper-triglyceridemia, and enlarged gallbladder volumes 8. These mice also have dramatically reduced gallbladder contractility to neurotransmitter stimulation in an in-vitro muscle bath 8 that is restored with leptin administration and glucose normalization 9. Heterozygous leptin-deficient (Lepob + / − ) mice are pheno-typically lean, not diabetic, and have slightly enlarged gallbladders. They have gallbladder motility that is less than control mice, but greater than the leptin-deficient Lepob or the leptin-resistant Lepdb mice 10. We have also shown that Agouti yellow (AY) mice which are overweight, but have normal leptin physiology and slightly elevated serum sugars, have gallbladder motility that resembles the C57 control mice 5.
Because of the similar findings of gallbladder enlargement and dysmotility of the leptin-deficient Lepob and both the Lepdb and Rotund leptin-resistant mice, leptin or obesity per se may not be directly responsible for these effects. Bouchard etal. reported that a dysfunctional leptin mechanism may indeed be protective against gallstone formation because of relatively normal biliary lipids 11. Further analysis of our gallbladder motility studies revealed that decreasing gallbladder contractility correlated significantly with both increasing serum glucose and triglyceride levels as well as body weight 10. Thus, hyperglycemia or hyperlipidemia maybe the link between obesity, leptin, and cholesterol gallstone disease. In fact, a recent study has demonstrated that while consumption of a high fat diet increases resistin, a hormone known to increase insulin resistance, leptin activation of the long leptin receptor ameliorates insulin resistance by downregulation of resistin 12. These authors also demonstrated that leptin administration to Lepdb animals did not down-regulate resistin and concluded that a functional long form of the leptin receptor was required 12. These findings also suggest that leptin plays a role in controlling diabetes, and these leptin dysfunctional mice may have gallbladder dysmotility as a consequence of their diabetes.
Gallbladder contraction is initiated by depolarization of intrinsic cholinergic neurons as well as by direct binding of neurotransmitters to myocyte surface receptors 13,14,15. The cholinergic ganglionic plexus is intrinsic to the gallbladder, lying between the serosa and muscle layers 15. These postganglionic nerves are present in the whole organ muscle bath, and prior studies have shown that cholecystokinin can readily access the subserosal gallbladder ganglia and activate the intrinsic cholinergic postganglionic nerves 15,16. Acetylcholine, which is released from the cholinergic nerves upon depolarization, causes autoexitation of the nerves and also potentiates contraction 17. Because CCK and ACh act via a neuronal mechanism, diabetic neuropathy may be an explanation for the abnormal gallbladder motility observed in Lepob, Lepdb, and Rotund mice.
In addition to neuronally activated gallbladder contraction, direct binding of neurotransmitters to myocyte surface muscarinic 17 and CCK-A 13 receptors also initiates gallbladder contraction. We have previously demonstrated that gallbladder myocytes from both the leptin-deficient Lepob mice and the leptin-resistant Lepdb mice are foreshortened, perhaps due to water loss from hyperglycemia 18. In addition, the myocytes from Lepob and Lepdb mice have a reduced response to CCK compared to lean control myocytes 18.
Our observations with the Lepob, Lepdb, and Rotund mice are also consistent with many gallbladder imaging studies in human diabetic patients which have reported that these patients have larger resting gallbladder volumes 19,20,21 and reduced contraction in response to a meal 19,20. We also have recently demonstrated that non-obese diabetic NOD mice, which are insulin deficient, have enlarged gallbladders with gallbladder dysmotility which worsens with progression of diabetes 22. Diabetes may affect, in part, alterations in the density or sensitivity of ACh or CCK receptors, or may alter access to these receptors. Advanced glycation end products (AGEP) occur when elevated sugars react non-enzymatically and cause covalent crosslinking of collagens and protein matrix 23 and may also inhibit gallbladder contractility by stiffening gallbladder myocytes or connective tissue, or by preventing egress of CCK to the gallbladder.
In addition to hyperglycemia, we have demonstrated that decreasing gallbladder motility correlates with increasing hypertriglyceridemia 10. Hypertyglyceri-demia often occurs in diabetes as AGEP can cause oxidation of low-density lipoproteins (LDL) and prevent recognition by the LDL receptor 23. Whether alone, or in conjunction with diabetes, elevated serum fats may promote free radical formation and inflammation in the gallbladder wall, interfering with contraction. In theory, elevated serum lipids may also influence gallbladder bile composition and gallstone formation.
Previous studies with the Lepob and Lepdb mice demonstrate that their bile is unsaturated with cholesterol on a standard low cholesterol diet 24,25. However, with consumption of a high cholesterol diet, similar to the diet consumed by many obese humans, gallbladder bile of the Lepob and Lepdb mice becomes nearly saturated with cholesterol 10,26. In addition, bile from the Lepob mice has faster cholesterol crystallization and growth when compared to control mice 24, while Lepdb mice have prolonged crystal observation times, and decreased crystal growth and mass 26. Because of the small numbers of Rotund animals available, we were not able to perform bile or crystal analysis, but we would speculate that their bile composition and physiology would be similar to the Lepdb mice.
As the human population becomes more obese and suffers the comorbidities associated with obesity, cholesterol gallstone disease will also pose a larger healthcare problem. Our studies suggest that the relationship between obesity and gallstone disease is indirectly influenced by leptin and its receptors, and the direct effects may be mediated by diabetes and/or lipid disorders. If present trends continue, elucidation of the exact mechanisms for cholesterol gallstone formation will become an even more important goal.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Nakeeb A, Comouzzie AG, Martin L, Sonnenberg GE, Swartz-Basile DA, Kissebah AH, Pitt HA. Gallstones: genetics versus environment. Ann Surg. 2002;235:842. doi: 10.1097/00000658-200206000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 4.Vaisse C, Halass JL, Horvath CM, Darnell JE, Jr, Stoffel N, Friedman JM. Leptin activation of Stat 3 in the hypothalamus of wildtype and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 5.Tran KQ, Swartz-Basile DA, Nakeeb A, Pitt HA. Gallbladder motility in Agouti-yellow and leptin-resistant obese mice. J Surg Res. 2003;113:56–61. doi: 10.1016/s0022-4804(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Taylor PN, Young D, Karst SY, Nishina PM, Naggert JK. New leptin receptor mutations in mice: Leprdb-rtnd, Leprdb-dmpg, and Leprdb-rlpy. J Nutr. 2003;133(5):1265–71. doi: 10.1093/jn/133.5.1265. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q, Mak KM, Ren C, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells. J Biol Chem. 2004;279(6):4292–304. doi: 10.1074/jbc.M308351200. [DOI] [PubMed] [Google Scholar]
- 8.Goldblatt MI, Swartz-Basile DA, Svatek CL, Nakeeb A, Pitt HA. Decreased gallbladder response in leptin-deficient obese mice. J Gastrointest Surg. 2002;6:438–42. doi: 10.1016/s1091-255x(01)00046-4. [DOI] [PubMed] [Google Scholar]
- 9.Phillips J, Tran KQ, Goldblatt MI, Swartz-Basile DA, Nakeeb A, Pitt HA. Leptin ameliorates the gallbladder's response to neurotransmitters in congenitally obese mice. Gastroenterol. 2002;123:9A. [Google Scholar]
- 10.Tran KQ, Goldblatt MI, Swartz-Basile DA, Svatek CL, Nakeeb A, Pitt HA. Diabetes and hyperlipidemia correlate with gallbladder contractility in leptin-related murine obesity. J Gastrointest Surg. 2003;7:857–62. doi: 10.1007/s11605-003-0030-z. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard G, Johnson D, Carver T, Paigen B, Carey MC. Cholesterol gallstone formation in overweight mice establishes that obesity per se is not linked directly to cholelithiasis risk. JLipidRes. 2002;43:1105–13. doi: 10.1194/jlr.m200102-jlr200. [DOI] [PubMed] [Google Scholar]
- 12.Asensio C, Cettour-Rose P, Theander-Carrillo C, Rohner-JeanRenaud F, Muzzin P. Changes in glycemia by leptin administration or high-fat feeding in rodent models of obesity/type 2 diabetes suggest a link between resistin expression and control of glucose homeostasis. Endocrinology. 2004;145:2206–13. doi: 10.1210/en.2003-1679. [DOI] [PubMed] [Google Scholar]
- 13.Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J Nutr. 1994;124:1334S–9S. doi: 10.1093/jn/124.suppl_8.1334S. [DOI] [PubMed] [Google Scholar]
- 14.Yu P, Chen Q, Xiao Z, Harnett K, Biancani P, Behar J. Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am J Physiol. 1998;275:G203–9. doi: 10.1152/ajpgi.1998.275.2.G203. [DOI] [PubMed] [Google Scholar]
- 15.Mawe GM, Talmage EK, Cornbrooks EB, Gokin AP, Zhang L, Jennings LJ. Innervation of the gallbladder: Structure, neurochemical coding, and physiological properties of guinea pig gallbladder ganglia. Micro Res Tech. 1997;39:1–13. doi: 10.1002/(SICI)1097-0029(19971001)39:1<1::AID-JEMT1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Hanyu N, Dodds WJ, Layman RD, Hogan WJ, Chey WY, Takahashi I. Mechanism of cholecystokinin-induced contraction of the opossum gallbladder. Gastroenterology. 1990;98:1299–1306. doi: 10.1016/0016-5085(90)90348-5. [DOI] [PubMed] [Google Scholar]
- 17.Parkman HP, Pagano AP, Ryan JP. Subtypes of muscarinic receptors regulating gallbladder cholinergic contractions. Am J Physiol. 1999;276:G1243–9. doi: 10.1152/ajpgi.1999.276.5.G1243. [DOI] [PubMed] [Google Scholar]
- 18.Graewin SJ, Lee K-H, Kiely JM, Svatek CL, Nakeeb A, Pitt HA. Gallbladder myocytes are short and CCK-resistant in obese diabetic mice. Surgery. 2004;136:431–6. doi: 10.1016/j.surg.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Kayacetin E, Kisakol G, Kaya A, Akpinar Z. Real-time sono-graphy for screening of gallbladder motility in diabetic patients: Relation to autonomic and peripheral neuropathy. Neuro-endocrinology Letters. 2003;24:73–6. [PubMed] [Google Scholar]
- 20.Nakeeb A, Sonnenberg GE, Touzios J, Comuzzie AG, Kissebah AH, Pitt HA. Altered glucose metabolism is associated with impaired gallbladder motility. HPB. 2004;6((Suppl)):48–9. [Google Scholar]
- 21.Hahm JS, Park JY, Park KG, Ahn YH, Lee MH, Park KN. Gallbladder motility in diabetes mellitus using real time ultra-sonography. Am J Gastroenterol. 1996;91:2391–4. [PubMed] [Google Scholar]
- 22.Graewin SJ, Kiely JM, Lee K, Svatek CL, Nakeeb A, Pitt HA. Non-obese diabetic mice have diminished gallbladder motility and shortened crystal observation time. J Gastrointest Surg. 2004;8:824–30. doi: 10.1016/j.gassur.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 24.Goldblatt MI, Choi SH, Swartz-Basile DA, Nakeeb A, Pitt HA. Cholesterol crystal formation in congenitally obese mice. Surg Forum. 2000;51:1–2. [Google Scholar]
- 25.Tran KQ, Graewin SJ, Swartz-Basile DA, Nakeeb A, Svatek CL, Pitt HA. Leptin-resistant obese mice have paradoxically low biliary cholesterol saturation. Surg. 2003;134:372–7. doi: 10.1067/msy.2003.234. [DOI] [PubMed] [Google Scholar]
- 26.Graewin SJ, Lee K, Tran KQ, Goldblatt MI, Svatek CL, Nakeeb A, Pitt HA. Leptin-resistant obese mice do not form biliary crystals on a high cholesterol diet. J Surg Res. 2004;122:145–9. doi: 10.1016/j.jss.2004.04.012. [DOI] [PubMed] [Google Scholar]