Abstract
During the last three decades liver cell adenoma and liver cell adenomatosis have emerged as new clinical entities in hepato-logical practice due to the widespread use of oral contraceptives and increased imaging of the liver. On review of published series there is evidence that 10% of liver cell adenomas progress to hepatocellular carcinoma, diagnosis is best made by open or laparoscopic excision biopsy, and the preferred treatment modality is resection of the liver cell adenoma to prevent bleeding and malignant transformation. In liver cell adenomatosis, the association with oral contraceptive use is not as high as in solitary liver cell adenomas. The risk of malignant transformation is not increased compared with solitary liver cell adenomas. Treatment consists of close monitoring and imaging, resection of superficially located, large (>4 cm) or growing liver cell adenomas. Liver transplantation is the last resort in case of substantive concern about malignant transformation or for large, painful adenomas in liver cell adenomatosis after treatment attempts by liver resection.
Keywords: Liver cell adenoma, liver cell adenomatosis
The prevalence of patients with liver cell adenoma is increasingly seen within hepatology practice due to the widespread use of oestrogen-based oral contraceptives, and due to the increased use of cross-sectional imaging for a variety of unrelated reasons. Consequently many lesions are identified as incidental findings in asymptomatic patients. The clinical significance and natural history of these incidental solid liver lesions is not fully understood, and there is a need for an optimal management strategy in such patients.
Liver cell adenoma is a benign neoplasm of the liver that has significant aetiological association with the oral contraceptive pill in young women. Liver cell adenoma secondary to oestrogen/progestogen ingestion is usually solitary, but some people may develop several adenomas disseminated throughout the liver. This latter condition is known as liver cell adenomatosis, does not have the strong association with oestrogen or anabolic steroid ingestion, and affects males more readily 1.
This article presents the current knowledge and optimal therapeutic strategies for patients with solitary liver cell adenoma and liver cell adenomatosis.
Background
Liver cell adenoma is the most important benign epithelial tumour of the liver, and has an estimatedincidence of 3 per 1 000 000 per year 2. The annual incidence is substantially higher with long-term oral contraceptive use, estimated at 3–4 per 100 000 3, but may be less with newer oral contraceptives 4.
Liver cell adenoma was first described by Edmondson 5 in 1958 as an encapsulated liver tumour that does not contain bile ducts, when he identified two such lesions in 50 000 autopsies. In 1973, Baum 6 reported the important relationship between oral contraceptive use and the development of liver cell adenomas in seven patients. Several subsequent case series 7,8 in the 1970s supported the hypothesis of an association between the oral contraceptive pill and liver cell adenoma, and in 1976 Edmondson 9 published a case-control study giving further evidence of this association. The causal relationship between oral contraceptive medication and liver cell adenoma appears to be proportional to the hormonal dose and duration of medication 3,10,11, and is highest in women over 30 years of age after using oral contraceptives for more than 24 months. It is estimated the risk of developing an adenoma increases by a factor of 5 after 5 years, and by 25 after 9 years of oral contraceptive usage 11. Regression of the tumour may occur after cessation of oral contraceptive usage 12, and there are reports of progression to hepatocellular carcinoma many years after stopping oral contraceptives 13,14,15. Pregnancy appears to stimulate rapid growth in these lesions with risk of potentially fatal spontaneous rupture, and should be avoided in women of childbearing age 3.
Other possible aetiologies of liver cell adenoma include clomiphene 16, methyl testosterone 17, danazol 18, Klinefelter's syndrome 19, Types I, III and IV glycogen storage disease 20,21, and familial adenomatous polyposis 22.
Liver cell adenoma is usually a solitary nodule that may reach 30 cm in diameter. Macroscopically these lesions are smooth and soft on palpation, and range in colour from white to yellow to brown. On histological examination adenomas consist of cords of hepatocytes that have a high glycogen and fat content. The normal hepatic parenchymal architecture is lacking, with an absence of portal tracts and hepatic veins.
Liver cell adenomatosis is present in 10%–24% 23,24,25 of patients with liver cell adenoma and presents specific management difficulties. Liver cell adenomatosis was originally thought to affect males and females equally, but the most recent series report a female: male ratio of 7:1 and 15:1, respectively 26,27. There is a strong association between liver cell adenomatosis and glycogen storage disease, but the association of liver cell adenomatosis and oral contraceptive or androgenic steroid use 1 is uncertain.
Presentation and diagnosis
Symptomatic patients usually present with right upper quadrant pain secondary to bleeding within the liver cell adenoma. At initial presentation these symptoms are often attributed to cholecystitis, the most common diagnosis in this patient population. Liver function tests may be abnormal secondary to necrosis or haemorrhage, and alkaline phosphatase is often elevated in those with liver cell adenomatosis. Some present with an acute abdomen and life threatening haemorrhage secondary to an uncontained rupture and bleeding into the peritoneal cavity, but most have a more indolent clinical presentation.
Some liver cell adenomas are picked up incidentally during imaging studies of the liver or noted during laparoscopic cholecystectomy. The differential diagnosis includes focal nodular hyperplasia, a benign liver lesion of vascular origin, and well-differentiated hepatocellular carcinoma. These two lesions can be difficult to differentiate from adenoma and remain the diagnostic challenge as they have different therapeutic implications. Patients with focal nodular hyperplasia are less likely to be symptomatic or have deranged liver function tests 28,29.
The ultrasonographic features of liver cell adenomas are non-specific and may appear iso-, hypo-, or hyperechoic. The classical appearance is of a well-demarcated hyper-echoic mass, but central necrosis or haemorrhage gives rise to heterogeneous echogenicity that simulates that of focal nodular hyperplasia 30. The CT appearances of adenoma may be quite variable, but the classical lesion characteristics are best appreciated with multi-phase helical CT scanning. Liver cell adenoma shows early phase peripheral contrast enhancement and subsequent centripetal contrast enhancement 31. The pattern of perfusion starting at the periphery can be demonstrated on angiography and has been used to differentiate liver cell adenomas from focal nodular hyperplasia where the vascular supply arises centrally from a feeding artery leading to rapid filling of the suprahepatic vein (‘spoke wheel’ appearance) 32.
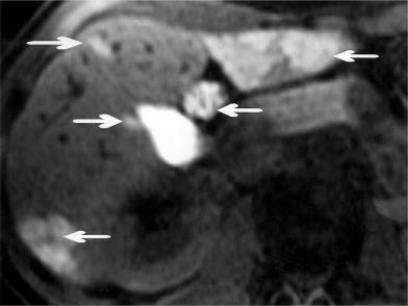
Magnetic resonance imaging has the optimal sensitivity for lesion detection, but again characterization of the lesion can be variable. Ultra-fast sequences with breath-holding and gadolinium contrast provide most information 27,33,34, and surveillance of patients with liver cell adenomatosis is highly effective using a delayed teslascan 24 hours after injection of gadolinium (see Figure 1). Even with these advances it may be impossible to differentiate liver cell adenoma from focal nodular hyperplasia or hepatocellular carcinoma, and a biopsy is then essential for histological clarification.
Figure 1. .
Teslascan MRI at 24 hours after injection: uptake of contrast in left lobe and lateral aspect of right lobe by multiple adenomas (arrows).
Lesion biopsy
Percutaneous biopsy of a liver lesion is not to be recommended in young fit patients as this can induce bleeding and tumour dissemination, does not exclude malignancy if normal tissue is found, and may be inaccurate. In a study by Charny et al., only 11 of 30 biopsies were accurate 35. There are numerous case reports of women using oral contraceptives who develop hepatocellular carcinomas, but preoperative or operative liver needle biopsies reveal liver cell adenomas only 14,36,37,38,39 (Table I). Excision biopsy of liver lesions either by open surgery, or laparoscopically 40, is the gold standard method for diagnosis. Even with tumour tissue, accurate microscopic differentiation between benign liver cell adenomas and hepatocellular carcinoma can be difficult. QBend 10- and erbB2-immunostaining 41,42, comparative genomic in situ hybridization 43, and fluorescence in-situ hybridization 44 may differentiate between hepatic adenoma and hepatocellular carcinoma but have not yet entered routine clinical practice.
Table I. Malignant transformation of liver adenomas.
| Length of OC use | αFP | Liver biopsy | Specimen analysis/particularities | |
|---|---|---|---|---|
| Davis M, 1975 62 | 2 years | normal | – | Well-differentiated hepatocellular carcinoma (HCC) in adenoma |
| Pryvor AC, 1977 36 | 12 years | normal | liver adenoma | Well-differentiated HCC in adenoma |
| TeslukH, 1981 14 | 5 years | normal | liver adenoma | Adenoma regressed after OC stop, but did not disappear, 3 years later development of HCC. |
| Gordon SC, 1986 15 | 14 years | normal | – | Adenoma regressed after stop of OC; 5 years later development of HCC |
| GyorffyEJ, 1989 63 | 19 years | initially normal, later on elevated | – | Initial percutaneous liver biopsy: liver adenoma Repeat biopsy 3 years later: HCC |
| KorulaJ, 1991 37 | 21 years | normal | liver adenoma | Well-differentiated HCC in adenoma |
| Ferrell DL, 1993 38 | for 6 months, 5 years before presentation | unknown | liver adenoma | Well-differentiated HCC within multilobular adenoma |
| Herman P, 1994 39 | 15 years | unknown | – | Well-differentiated HCC in adenoma |
| 20 years | unknown | benign | Well-differentiated HCC in adenoma | |
| PerretAG, 1996 64 | 3 years | normal | – | Disseminated foci of HCC in 14 cm multilobular adenoma |
| YeMQ, 1999 65 | 25 years | normal | – | Well-differentiated HCC in adenoma |
OC = oral contraceptives. HCC = hepatocellular carcinoma. αFP = α-fetoprotein.
Complications and management of solitary liver cell adenoma
Haemorrhage
This is a well-recognized complication of solitary liver cell adenoma 45,46,47 and is often the cause of pain in symptomatic patients. The morphology of adenomas with their extensive proliferation of blood-filled sinusoids, supplied by high-pressure arterial flow, and poor soft tissue support, makes them prone to bleed. The frequency of this complication is difficult to estimate, as this data is omitted in most case series, and many patients are advised to undergo resection of solitary liver cell adenoma at diagnosis to prevent a later presentation with tumour bleeding.
The risk of spontaneous bleeding into, or from, a ruptured adenoma, is likely to be between 20% and 40%. Selected series have demonstrated evidence of bleeding on microscopy in 4 of 10 patients 23, 3 of 15 patients on ultrasound, and 4 of 10 patients on contrast CT 28. Most bleeding is contained and does not present a life-threatening event, but bleeding from a liver cell adenoma can be fatal 48, and in the series reported by Rooks, 6 of 79 patients (8%) with liver cell adenoma died due to bleeding 3.
It has been suggested that adenomas greater than 5 cm in diameter should be excised as they are at increased risk of bleeding 49. However, Flowers 50 and Minami 51 reported patients with massive bleeding from adenomas of only 3.5 cm diameter, with one patient fatality in the postoperative period. In contrast, other patients with large adenomas have been observed for prolonged periods without evidence of bleeding. Currently there is no evidence to correlate risk of bleeding with size or number of liver cell adenomas.
There are numerous reports of bleeding liver cell adenomas during pregnancy and the puerperium, and women with known liver cell adenomas should be strongly advised not to become pregnant 3,52,53,54,55,56,57,58,59. Excision of a liver cell adenoma has been successfully performed during the second trimester 52. Given this risk and the unpredictable natural history of solitary liver cell adenoma with respect to bleeding, the majority of patients should be advised to undergo surgical resection.
Patients who present with bleeding or rupture will require surgical resection. However, the extent of resection and mortality can be reduced by delaying surgery to allow stabilization of the patient. Interventional radiological procedures such as hepatic artery embolization are a useful adjunct in this situation 60. Depending on the severity of bleeding the options are emergency excision on presentation, elective excision after haemodynamic stabilization or conservative management with delayed excision 61.
Malignant transformation in solitary liver cell adenoma
There are numerous reports of malignant transformation of liver cell adenoma to hepatocellular carcinoma 14,15,37,38,39,62,63,64,65. Most reported cases are associated with oral contraceptive use for a prolonged period of time. α-fetoprotein levels are often within normal range in these patients and thus a poor indicator of tumour progression (Table I).
Most cases of hepatocellular carcinoma develop at the site of the liver cell adenoma lending support to the hypothesis of a progressive adenoma-carcinoma sequence. However, some pathologists debate the malignant potential of liver cell adenomas and consider them as the end-stage of hepatocyte proliferation induced by anabolic steroids 66. The international working party of hepatocellular lesions takes a cautious view on the question of malignant potential of liver cell adenomas and states that ‘malignant progression has been reported, but is rare’ 67. Of the 14 series on liver cell adenoma reported to date 24,28,29,50,60,61,68,69,70,71,72,73,74,75, 10 series 29,60,61,69,70,71,72,73,74,75 have reported progression or de novo development of hepatocellular carcinoma within the liver cell adenoma and this occurs with a frequency of 1:12 to 1:9 (i.e. 8%–11%) (Table II). Five of the 10 series 60,67,69,70,73 specified follow-up ranging between 16–81 months. One patient died from progressive hepatocellular carcinoma 18 months following resection 69. There have been no reports of recurrence or progression to liver cell carcinoma in the other patients.
Table II. . Adenoma series.
| Adenoma series | Patients | Hepatocellular carcinoma | Male | Rupture | Bleeding | Oral contraceptive use |
|---|---|---|---|---|---|---|
| Weil R, 1979 68 | 8 | 0 | 1 | |||
| KerlinP, 1983 69 | 23 | 2 | 2 | 19:23 | ||
| LeeseT, 1988 24 | 18 | 0 | 16: 18 | |||
| Flowers BF, 1990 50 | 6 | 0 | 1 | 2 | 5:5 | |
| BelghitiJ, 1993 70 | 13 | 1 | ||||
| Cherqui D, 1995 28 | 6 | 0 | 2 | CT3:6 MRI 3:5 | 5:6 | |
| Nagorney DM, 1995 71 | 24 | 2 | 2 | 2 | 2 | 9:22 |
| Ault GT, 1996 60 | 11 | 3 | 1 | 9:10 | ||
| DeCarlis L, 1997 72 | 19 | 2 | 17: 19 | |||
| Weimann A, 1997 29 | 44 | 3 | 1 | 6 | ||
| Ichikawa T, 2000 73 | 25 | 2 | 4 | CT11:44 | 12:21 | |
| Reddy KR, 2001 74 | 25 | 1 | 3 | 22:25 | ||
| Charny CK, 2001 75 | 12 | 1 | 2 | |||
| MariniP, 2002 61 | 7 | 1 | 6 | 7 | 7:7 |
There is a real risk of malignant transformation in the order of 10%, and this should be seriously considered when advising patients.
Tao states that oral contraceptive-induced liver cell adenomas are reversible if oral contraceptives are discontinued within a certain time period, and that after prolonged oral contraceptive usage dysplastic foci develop within the liver cell adenoma that progress to hepatocellular carcinomas 76.
Fully developed cancers have extensive genomic damage as the result of their genetic instability (‘bystander effect’) 77. Pre-cancerous and benign tumours have less genetic alterations. Liver cell adenomas attracted the attention of genetic research in recent years to identify the initial critical genetic event in the development of hepatocellular carcinoma. Results are yet inconclusive and summarized in Table III.
Table III. Genome analysis of liver adenomas.
| Analysis | Interpretation | Results | |
|---|---|---|---|
| Brunt EM, 1992 78 | Immunohistochemistry of c-erbB-2 oncopeptide | Over-expressed in epithelial neoplasms | Negative in 2/2 liver adenomas |
| Ding SF, 1992 79 | Southern blot of DNA from liver adenoma and HCC in one patient | Chromosome 17p13 loss comprising the p53 tumour suppressor gene in hepatocellular carcinoma, but not in liver adenoma | |
| Nasarek A, 1995 80 | Fluorescence in-situ hybridization of chromosomes 1 and 8 | Common aberrations in hepatocellular carcinomas (HCC) | Negative |
| Gaffey MJ, 1996 81 | PCR of HUMARA gene | Detects x-chromosome inactivation | Clonality in 2/2 liver adenomas |
| Paradis V, 1997 82 | PCR of HUMARA gene | Detects x-chromosome inactivation | Clonality in 6/7 liver adenomas |
| Strassburg CP, 1997 83 | Northern and Western blot analysis of UGT1A locus | UGT1A-locus downregulated in liver adenoma and HCC | |
| D'Errico AD, 1998 84 | IHC of CK 8, 18, 7, 19 | Hepatocellular differentiation CK 8, 18 Biliary differentiation CK 8, 18, 7, 19 | CK 8, 18 positive in 10/10 liver adenomas CK 7, 19 negative in 10/10 liver adenomas |
| De Boer CJ, 1999 85 | IHC for Ep-Cam | Marker of early neoplasia in adult cells | Negative in 2/2 liver adenomas |
| Libbrecht L, 2001 23 | IHC for CK 7, 8, 18, 19, chromogranin A, OV-6 and neural cell adhesion molecule (NCAM) as markers for hepatic progenitor cells | Hepatic progenitor cells present in 5/10 adenomas | Hepatic progenitor cells have potential role in hepatocarcinogenesis |
| Tannapfel A, 2002 86 | PCR and fluorescence in-situ hybridization FISH for INK4-ARF inactivation and microdeletion of p14ARFand p16INK4a | INK4a-ARF (CDKN2A) locus on chromosome 9p21 encodes the tumour suppressor proteins p14ARF and p16INK4a and is frequently inactivated in many human cancers | Alterations of p14ARF and p16INK4a in 3/25 and 6/25 liver adenomas, respectively, not necessarily associated with malignancy |
| Takayasu H, 2002 87 | IHC for β-catenin, PCR of β-catenin gene | Genes for β-catenin involved in Wnt pathway, Wnt pathway activated in HCC | Nuclear accumulation of β-catenin in 2/2 liver adenomas, b-catenin gene mutation in 1/1 liver adenoma |
| Torbenson M, 2002 88 | IHC for b-catenin and PCR of cluster region of adenomatosis polyposis coli protein. Loss of heterozygosity analysis of chromosome 5q | Genes for β-catenin and APC involved in Wnt pathway, Wnt pathway activated in HCC | Nuclear accumulation of β-catenin in 7/15 liver adenomas No mutation of b-catenin- and APC-gene, no LOH of 5q |
| Chen YJ, 2002 89 |
Comparative genomic hybridization |
Frequent gains on chromosomal arms 1q (50%), 17q (50%), 1p (38%) and 11q (38%) |
Genome alterations of liver adenomas coincide with those of HCC |
IHC = immunohistochemistry. PCR = polymerase chain reaction. CK = cytokeratins.
The etiological role of oral contraceptives in inducing liver cell adenoma is beyond doubt, and many studies have shown regression and even complete resolution of adenomas after cessation of the oral contraceptive pill 90,91,92,93,94,95,96,97. This observation is not consistent, as others have not noted any change in the size of existing liver cell adenomas after discontinuation of oral contraceptives 98,99. It probably correlates with the observation that the detection of hormone receptors in liver cell adenomas varies between 26% and 73% 100,101. Discontinuation of oral contraceptives before surgery is worthwhile, as this may facilitate adenoma regression and reduce the extent of liver resection.
It is important to note that even complete resolution of the adenoma does not prevent the late occurrence of hepatocellular carcinoma, as this has been observed 3–5 years after cessation of oral contraceptive usage and regression of the adenoma 14,15. In contrast, there are no reports of de novo adenoma occurrence or formation of hepatocellular carcinoma after primary resection of solitary liver cell adenoma. This provides a strong argument in favour of liver resection as the primary treatment for solitary liver cell adenoma.
Resection of solitary liver cell adenoma
Prevention of bleeding and/or malignant transformation is guaranteed with surgical resection, but there is morbidity and mortality associated with surgical resection. Depending on experience, the morbidity associated with liver resection for benign tumours ranges from 10% to 27%, and mortality from 0% to 3% 49,70.
Despite the risk associated with surgery, we strongly favour surgical resection of solitary liver cell adenoma for four reasons. Firstly, the risk of malignant transformation is substantial (around 10%), and regression of liver cell adenomas after stopping oral contraceptives does not prevent late malignant transformation. Secondly, bleeding or rupture of a liver cell adenoma is common and unpredictable, occurring in 30%–50% of such lesions. This bleeding is unrelated to lesion size or site, and may be fatal. Thirdly, it may be impossible to differentiate on radiology alone between an adenoma and a well-differentiated hepatocellular carcinoma. This necessitates resection and histological analysis to reassure patient and clinician. Finally, surgical resection provides a guaranteed long-term cure and no risk of tumour recurrence. Given these facts, surgical resection is presently the optimal treatment for all patients that present with solitary liver cell adenoma.
Liver cell adenomatosis
The same recommendation cannot be made for those who present with multiple adenomas within the liver. The clinical entity of liver cell adenomatosis was first proposed by Flejou et al. in 1985, who described 5 patients with multiple adenomas of the liver and collated this experience with 8 similar previous case reports. The characteristic features of liver cell adenomatosis proposed at that time were (a) arbitrarily more than 10 tumor nodules in the liver, (b) equal male/female distribution, (c) no association with oral contraceptives, (d) increased levels of serum alkaline phosphatase and γ-glutamyl transpeptidase 1.
Multiple adenomas occur in approximately 10%–24% of all patients with liver cell adenomas 23,24,25, and the reason for this remains obscure. Given the disparate and extensive distribution of lesions in liver cell adenomatosis, these patients present a more difficult management problem, as targeted adenoma excision as for solitary liver cell adenoma is not a practical option. Chiche et al. have suggested two distinct patterns of disease with liver cell adenomatosis 26. The ‘massive type’ presents with gross hepatomegaly, a deformed liver contour, and contains many large tumour nodules ranging from 2–10 cm in diameter. The massive type may be rapidly progressive and presents a particular therapeutic challenge. The ‘multifocal type’ contains many adenomas up to 4 cm in diameter but the liver contour is not deformed or enlarged. These patients are unlikely to have symptoms and appear to have a less aggressive clinical course.
Risk of bleeding
As with solitary liver cell adenoma, patients with liver cell adenomatosis frequently present with right upper abdominal pain attributed to bleeding into the adenoma. As these patients have multiple lesions the risk of bleeding is substantially higher, occurring in 46% of patients in the series by Flejou et al.1. As it is not possible to resect all adenomatous lesions (except by liver transplantation), prophylactic liver resection to prevent bleeding must be targeted to those lesions at greatest risk of causing life-threatening haemorrhage. Large lesions >4 cm in diameter, and sub-capsular lesions that may bleed into the peritoneal cavity, should be considered for resection to prevent bleeding 102. Of the 81 patients with liver cell adenomatosis described, 15 (18%) have presented with intra-abdominal bleeding 26,69,103,104,105, and two patients died on presentation from intraperitoneal haemorrhage 26,106.
Risk of malignancy
Chiche et al. challenge the malignant potential of adenomas in patients with liver cell adenomatosis 26, primarily as data in this regard are scant. However, six patients with undisputable liver cell adenomatosis are known to have undergone malignant transformation 24,25,27,107,108, with four of the six patients male. The original observation that liver cell adenomatosis affected men and women equally is no longer valid, as the majority of affected individuals (74%) to date are female 26. Thus, male sex may be a risk factor for malignant transformation in liver cell adenomatosis. The 81 liver cell adenomatosis patients currently documented in the literature have an average documented follow-up of 57 months (range 3–180 months), at which time-point 41 patients have stable disease, 34 patients have progression of disease with increased size and/or number of lesions, and 6 (7%) have developed malignancy. At present there is no evidence to suggest that the risk of malignant transformation is increased in liver cell adenomatosis compared with that of solitary adenoma.
Liver transplantation for liver cell adenomatosis
The only potential cure for liver cell adenomatosis is liver transplantation, and therefore some have advised organ transplantation for this condition 69,70,109,110. The benefit of liver transplantation to prevent bleeding or cancer in these young patients has to be balanced against the potential risk of transplantation. Perioperative mortality of liver transplantation is less than 1%, and 5- and 8-year survival are about 66% and 61% respectively. Most deaths (65%) occur within the first 6 months 111.
In the longer term, up to 11.5% of liver recipients have been reported to develop de novo tumours on immunosuppression 112, renal failure occurs in 10% after 10 years 113, and hypertension in more than 5 0% 114. The rate of leakage or stenosis of the biliary anastomosis varies between 15% and 33% 115. Rejection occurs in 3%–5% 116,117.
Of the 17 cases of liver transplantation for liver cell adenomatosis in the literature 24,27,108,118,119,120, outcome and survival are known for 10 patients (see Table IV). Average length of follow-up is 87 months (36–145 months). There was one death in the immediate postoperative period, and three patients have developed hepatocellular carcinomas in the transplanted liver or developed lung metastasis 27,108. Two of these three patients did not have any evidence of malignancy in their native liver. Hepato-cellular carcinoma in the transplanted organ became apparent 9 and 12 years after liver transplantation. This casts considerable doubt on the indication for transplantation in liver cell adenomatosis to prevent malignant transformation, as malignant progression can still occur despite transplantation.
Table IV. Results of liver transplantation for liver adenomatosis.
| Age (years)/Sex | Indication | Interval diagnosis—transplantation | Previous liver surgery unknown | αFP | Survival | Status | |
|---|---|---|---|---|---|---|---|
| Leese T, 1988 24 | 21/M | unknown | unknown | unknown | unknown | ||
| 14/M | 5 years | left lobectomy | ×105 | 13 months | alive | ||
| Tepetes K, 1995 119 | 10/F | 3 years | explorat.laparotomy×2 | 65 months | alive | ||
| 17/F | 2 years | portocaval shunt | 145 months | alive | |||
| 20/F | 5 years | segmentectomy | 101 months | alive | |||
| 31/F | 2 years | segmentectomy | 130 months | alive | |||
| 35/F | 15 years | explorat.laparotomy×2 | 36 months | alive | |||
| 43/F | 6 months | trisegmentectomy | 10 days | died of necrotizing pancreatitis | |||
| 46/F | 1 year | trisegmentectomy | 12 years | died of HCC 12 years after transplantation | |||
| Chiappa A, 1999 120 | 44/F | Progression of adenomatosis—liver failure | 13 months | wedge excision of one adenoma | Normal | unknown | unknown |
| Grazioli L, 2000 27 | 36/M | unknown | unknown | Normal | unknown | unknown | |
| 35/F | unknown | unknown | unknown | unknown | |||
| 19/F | unknown | unknown | unknown | unknown | |||
| 43/F | unknown | unknown | unknown | unknown | |||
| 46/F | 1 year | trisegmentectomy | 12 years | died of HCC 12 years after transplantation | |||
| Penna C, 2001 J Chir 118 | 20/M | Progression liver adenomatosis—malignancy | 5 years | Elevated | 10 years | live | |
| Yunta PJ, 2001 108 | 23/F | Progression liver adenomatosis—malignancy | diagnosis after transplantation | Elevated | 27 months | alive with HCC in transplant liver |
Management of liver cell adenomatosis
Given the morbidity and mortality associated with liver transplantation, current world experience would support a more conservative approach as the optimal initial management strategy. Patients should be entered into a surveillance program that includes regular (annual) CT or MRI scanning, and frequent serum alpha-fetoprotein measurement to detect progression of disease (increased lesion size) and/or malignant transformation.
All female patients should be advised to stop hormone medication (e.g. oral contraceptives, hormone replacement therapy), and also ensure that they prevent further pregnancies. Patients presenting with intraperitoneal bleeding require laparotomy and resection of the bleeding tumour.
Those who have the massive form of liver cell adenomatosis may have a preponderance of large lesions within a single lobe, and are best managed by hemi-hepatectomy. Patients with multifocal liver cell adenomatosis should be monitored with regular liver imaging. Progression of disease with large subcapsular adenomas (>4 cm), concern of malignant transformation, and increasing symptoms are indications for resection in multifocal liver cell adenomatosis. Resection is the preferable option unless technically impossible. Orthotopic liver transplantation should be considered only as a last resort. Indications where transplantation is considered may include rise in serum alpha-fetoprotein, concern about malignant transformation on imaging, and symptomatic patients with marked hepatomegaly and a history of repeated adenoma complications 24,26.
Conclusion
Liver cell adenoma is the most important benign tumour of the liver because of its frequency and potential for complications which are life threatening: bleeding and malignant transformation. Despite significant progress in imaging, definite diagnosis is by excision biopsy.
Discontinuation of oral contraceptives is often associated with regression of the adenoma, but there is still a risk of malignant transformation. Because of the risks of bleeding and malignant transformation surgical excision is the preferred treatment option for solitary liver cell adenomas.
Liver cell adenomatosis provides a clinical challenge. Symptomatic tumours, which are accessible to surgery, should be resected. The role of transplantation remains unclear. Other treatment modalities such as embolization of bleeding tumours and radiofrequency ablation may be useful adjuncts in selected cases.
Acknowledgements
We would like to thank Dr Sue Taylor for her help with the manuscript.
References
- 1.Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, Benhamou JP. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89(5):1132–8. [PubMed] [Google Scholar]
- 2.Wittekind C. Hermanek P, Gospodarowicz MK, Henson DE, et al. Springer; Berlin: 1995. Hepatocellular carcinoma and cholangiocarcinoma, Prognostic factors in cancer; pp. 88–93. [Google Scholar]
- 3.Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma—the role of oral contraceptive use. JAMA. 1979;242(7):644–8. [PubMed] [Google Scholar]
- 4.Heinemann LA, Weimann A, Gerken G, Thiel C, Schlaud M, Do Minh T. Modern oral contraceptive use and benign liver tumors: the German benign liver tumor-case control study. Eur J Contracept Reprod Health Care. 1998;3:194–200. doi: 10.3109/13625189809167253. [DOI] [PubMed] [Google Scholar]
- 5.Edmondson HA. Tumors of the liver and intrahepatic bile ducts. Section 7, fascicle 25, Atlas of Tumor Pathology. Washington: Armed Forces Institute of Pathology; 1958. [Google Scholar]
- 6.Baum JK, Holtz F, Bookstein JJ, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–9. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 7.Klatskin G. Hepatic tumors: possible relationship to use of oral contraceptives. Gastroenterology. 1977;73:386–94. [PubMed] [Google Scholar]
- 8.Horvath E, Kovacs K, Ross RC. Benign hepatoma in a young woman on contraceptive steroids. Lancet. 1974;1:357–8. doi: 10.1016/s0140-6736(74)93111-0. [DOI] [PubMed] [Google Scholar]
- 9.Edmonson HA, Henderson B, Benton B. Liver cell adenomas association with use of oral contraceptives. N Engl J Med. 1976;294:470–2. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 10.Nime F, Pickren JW, Vana J, Aronoff BL, Baker HW, Murphy GP. The histology of liver tumors in oral contraceptive users observed during a national survey by the American College of Surgeons Commission on Cancer. Cancer. 1979;44(4):1481–9. doi: 10.1002/1097-0142(197910)44:4<1481::aid-cncr2820440443>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg L. The risk of liver neoplasia in relation to combined oral contraceptive use. Contraception. 1991;43(6):643–52. doi: 10.1016/0010-7824(91)90007-3. [DOI] [PubMed] [Google Scholar]
- 12.Kawakatsu M, Vilgrain V, Erlinger S, Nahum H. Disappearance of liver cell adenoma: CT and MR imaging. Abdom Imaging. 1997;22(3):274–6. doi: 10.1007/s002619900188. [DOI] [PubMed] [Google Scholar]
- 13.Marks WH, Thompson N, Appleman H. Failure of hepatic adenomas (HCA) to regress after discontinuance of oral contraceptives. An association with focal nodular hyperplasia (FNH) and uterine leiomyoma. Ann Surg. 1988;208(2):190–5. doi: 10.1097/00000658-198808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesluk H, Lawrie J. Hepatocellular adenoma. Its transformation to carcinoma in a user of oral contraceptives. Arch Path Lab Med. 1981;105(6):296–9. [PubMed] [Google Scholar]
- 15.Gordon SC, Reddy KR, Livingstone AS, Jeffers LJ, Schiff ER. Resolution of a contraceptive-steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Int Med. 1986;105(4):547–9. doi: 10.7326/0003-4819-105-4-547. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco D, Barrachina M, Prieto M, Berenguer J. Clomiphene citrate and liver-cell adenoma. N Engl J Med. 1984;310(7):1120–1. doi: 10.1056/NEJM198404263101716. [DOI] [PubMed] [Google Scholar]
- 17.Coombes GB, Reiser J, Paradinas FJ, Burn I. An androgen-associated hepatic adenoma in a trans-sexual. Brit J Surg. 1978;65(2):869–70. doi: 10.1002/bjs.1800651212. [DOI] [PubMed] [Google Scholar]
- 18.Fermand JP, Levy Y, Bouscary D, D'Agay MF, Clot P, Frija J, Brouet JC. Danazol-induced hepatocellular adenoma. Am J Med. 1990;88(5):529–30. doi: 10.1016/0002-9343(90)90434-f. [DOI] [PubMed] [Google Scholar]
- 19.Beuers U, Richter WO, Ritter MM, Wiebecke B, Schwandt P. Klinefelter's syndrome and liver adenoma. J Clin Gastroenterol. 1991;13(2):214–6. doi: 10.1097/00004836-199104000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Alshak NS, Cocjin J, Podesta L, van de Velde R, Makowka L, Rosenthal P, Geller SA. Hepatocellular adenoma in glycogen storage disease type IV. Arch Path Lab Med. 1994;118(1):88–91. [PubMed] [Google Scholar]
- 21.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odievre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Ped Gastroenterol Nutr. 1997;24(3):276–9. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Bala S, Wunsch PH, Ballhausen WG. Childhood hepatocellular adenoma in familial adenomatous polyposis: mutations in adenomatous polyposis coli gene and p53. Gastroenterology. 1997;112(3):919–22. doi: 10.1053/gast.1997.v112.pm9041254. [DOI] [PubMed] [Google Scholar]
- 23.Libbrecht L, De Vos R, Cassiman D, Desmet V, Aerts R, Roskams T. Hepatic progenitor cells in hepatocellular adenomas. Am J Surg Path. 2001;25(11):1388–96. doi: 10.1097/00000478-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208(5):558–64. doi: 10.1097/00000658-198811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129(7):712–7. doi: 10.1001/archsurg.1994.01420310044007. [DOI] [PubMed] [Google Scholar]
- 26.Chiche L, Dao T, Salame E, Galais MP, Bouvard N, Schmutz G, Rousselot P, et al. Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231(1):74–81. doi: 10.1097/00000658-200001000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grazioli L, Federle MP, Ichikawa T, Balzano E, Nalesnik M, Madariaga J. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology. 2000;216(2):395–402. doi: 10.1148/radiology.216.2.r00jl38395. [DOI] [PubMed] [Google Scholar]
- 28.Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Metreau JM, Meignan M, Fagniez PL, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22(6):1674–81. [PubMed] [Google Scholar]
- 29.Weimann A, Ringe B, Klempnauer J, Lamesch P, Gratz KF, Prokop M, Maschek H, et al. Benign liver tumors: differential diagnosis and indications for surgery. World J Surg. 1997;21(9):983–90. doi: 10.1007/s002689900337. [DOI] [PubMed] [Google Scholar]
- 30.Golli M, Van Nhieu JT, Mathieu D, Zafrani ES, Cherqui D, Dhumeaux D, Vasile N, et al. Hepatocellular adenoma: color Doppler US and pathologic correlations. Radiology. 1994;190(3):741–4. doi: 10.1148/radiology.190.3.8115621. [DOI] [PubMed] [Google Scholar]
- 31.Van Hoe L, Baert AL, Gryspeerdt S, Vandenbosh G, Nevens F, Van Steenbergen W, Marchal G. Dual-phase helical CT of the liver: value of an early-phase acquisition in the differential diagnosis of noncystic focal lesions. AJR Am J Roentgenol. 1997;168(5):1185–92. doi: 10.2214/ajr.168.5.9129409. [DOI] [PubMed] [Google Scholar]
- 32.Welch TJ, Sheedy PF, Johnson CM, Stephens DH, Charboneau JW, Brown ML, May GR, et al. Focal nodular hyperpolasia and hepatic adenoma: comparison of angiography, CT, US and scintigraphy. Radiology. 1985;156:593–5. doi: 10.1148/radiology.156.3.3895291. [DOI] [PubMed] [Google Scholar]
- 33.Arrive L, Flejou JF, Vilgrain V. Hepatic adenoma: MR findings in 51 pathologically proved lesions. Radiology. 1994;193:507–12. doi: 10.1148/radiology.193.2.7972769. [DOI] [PubMed] [Google Scholar]
- 34.Paulson EK, McClelland JS, Washington K, Spritzer CE, Meyers WC, Baker ME. Hepatic adenoma: MR characteristics and correlation with pathological findings. Am J Roentgenol. 1994;163:113–6. doi: 10.2214/ajr.163.1.8010195. [DOI] [PubMed] [Google Scholar]
- 35.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, Blumgart LH. Management o 155 patients with benign liver tumours. Brit J Surg. 2001;88(6):808–13. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 36.Pryvor AC, Cohen RJ, Goldman RL. Hepatocellular carcinoma in a woman on long-term oral contraceptives. Cancer. 1977;40:884–8. doi: 10.1002/1097-0142(197708)40:2<884::aid-cncr2820400243>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Korula J, Yellin A, Kanel G, Campofiori G, Nichols P. Hepatocellular carcinoma coexisting with hepatic adenoma. Incidental discovery after long-term oral contraceptive use. West J Med. 1991;155:416–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrell LD. Hepatocellular carcinoma arising in a focus of multilobular adenoma. Am J Surg Pathol. 1993;17(5):525–9. doi: 10.1097/00000478-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Herman P, Machado MAC, Volpe P, Pugliese V, Vianna MR, Bacchella T, Machado MCC, et al. Transformacão de adenoma hepático em carcinoma hepatocelular em pacientes com uso prolongado de contraceptivo oral. Rev Hosp Clin Fac Med S Paulo. 1994;49(1):31–4. [PubMed] [Google Scholar]
- 40.Katkhouda N, Hurwitz M, Gugenheim J, Mavor E, Mason RJ, Waldrep DJ, Rivera RT, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229(4):460–6. doi: 10.1097/00000658-199904000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott FR, el-Refaie A, More L, Scheuer PJ, Dhillon AP. Hepatocellular carcinoma arising in an adenoma: value of QBend 10 immunostaining in diagnosis of liver cell carcinoma. Histopathology. 1996;28(5):472–4. doi: 10.1046/j.1365-2559.1996.t01-3-297345.x. [DOI] [PubMed] [Google Scholar]
- 42.Brunt EM, Swanson PE. Immunoreactivity for c-erbB-2 oncopeptide in benign and malignant disease of the liver. Am J Clin Pathol. 1992;97(5 Suppl 1):S53–61. [PubMed] [Google Scholar]
- 43.Wilkens L, Bredt M, Flemming P, Becker T, Kubicka S, Kreipe H. Differentiation of liver cell adenomata from well differentiated hepatocellular carcinomas by comparative genomic hybridisation. J Pathol. 2001;193:476–82. doi: 10.1002/path.825. [DOI] [PubMed] [Google Scholar]
- 44.Wilkens L, Bredt M, Flemming P, Becker T, Mengel M, von Wasiliewski R, Klempnauer J, et al. Diagnostic impact of fluorescence in situ hybridization in the differentiation of hepatocellular adenoma and well-differentiated hepatocellular carcinoma. J Mol Diag. 2001;3:68–73. doi: 10.1016/S1525-1578(10)60654-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adusumilli PS, Lee B, Parekh K, Dolgopolov S. Hemoperitoneum from spontaneous rupture of a liver cell adenoma in a male with hyperthyroidism. Am Surg. 2002;68(7):582–3. [PubMed] [Google Scholar]
- 46.Heeringa B, Sardi A. Bleeding hepatic adenoma: expectant treatment to limit the amount of liver resection. Am Surg. 2001;67(10):927–9. [PubMed] [Google Scholar]
- 47.Bein NN, Goldsmith HS. Recurrent massive haemorrhage from benign hepatic tumours secondary to oral contraceptives. Br J Surg 1977;(64):433–5. [DOI] [PubMed] [Google Scholar]
- 48.Contostavlos DL. Benign hepatomas and oral contraceptives. Lancet. 1973;24:1200. doi: 10.1016/s0140-6736(73)92956-5. [DOI] [PubMed] [Google Scholar]
- 49.Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Tilanus HW, IJzermans JN. Treatment of ruptured hepatocellular adenoma. Brit J Surg 2001;88(2):207–9. [DOI] [PubMed] [Google Scholar]
- 50.Flowers BF, McBurney RP, Vera SR. Ruptured hepatic adenoma. A spectrum of presentation and treatment. Am Surg. 1990;56(6):380–3. [PubMed] [Google Scholar]
- 51.Minami Y, Kudo M, Kawasaki T, Chung H, Matsui S, Kitano M, Suetomi Y, et al. Intrahepatic huge hematoma due to rupture of small hepatocellular adenoma: A case report. Hepat Res. 2002;23(2):145–51. doi: 10.1016/s1386-6346(01)00164-4. [DOI] [PubMed] [Google Scholar]
- 52.Terkivatan T, De Wilt JHW, De Man RA, Ijzermans JNM. Management of hepatocellular adenoma during pregnancy. Liver. 2000;20(2):186–7. doi: 10.1034/j.1600-0676.2000.020002186.x. [DOI] [PubMed] [Google Scholar]
- 53.Bis KA, Waxman G. Rupture of the liver associated with pregnancy: a review of the literature and report of two cases. Obstet Gynecol Surv. 1976;31:763–73. doi: 10.1097/00006254-197611000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Hayes D, Lamki H, Hunter IW. Hepatic-cell adenoma presenting with intraperitoneal haemorrhage in the puerperium. Br Med J. 1977;26:1394. doi: 10.1136/bmj.2.6099.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monks PL, Fryar BG, Biggs WW. Spontaneous rupture of an hepatic adenoma in pregnancy with survival of mother and fetus. Aust N Z J Obstet Gynaecol. 1986;26:155–7. doi: 10.1111/j.1479-828x.1986.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 56.Rosel H D, Baier A, Mesewinkel F. Exsanguination caused by liver cell adenoma and rupture of the hepatic capsule as cause of maternal death. Zentralbl Gynäkol. 1990;112:1363–7. [PubMed] [Google Scholar]
- 57.Estebe JP, Malledant Y, Giullont YM. Rupture spontane d'un adénome du foie pendant la grossesse. J Chir. 1988;125:654–6. [PubMed] [Google Scholar]
- 58.Tsang V, Halliday AW, Collier N, Benjamin IS, Blumgart I. Hepatic cell adenoma: spontaneous rupture during pregnancy. Dig Surg. 1989;6:86–7. [Google Scholar]
- 59.Rosales J, Avila E, Orrego M, Cajas AM, Zolezzi A. Hepatic adenoma rupture as a cause of bleeding in the third trimester of pregnancy: report of a case and review of the literature. Rev Gastroenterol Peru. 2001;21(4):312–5. [PubMed] [Google Scholar]
- 60.Ault GT, Wren SM, Ralls PW, Reynolds TB, Stain SC. Selective management of hepatic adenomas. Am Surg 2001Oct;67(10):927–9. [Google Scholar]
- 61.Marini P, Vilgrain V, Belghiti J. Management of spontaneous rupture of liver tumours. Dig Surg. 2002;19:109–13. doi: 10.1159/000052022. [DOI] [PubMed] [Google Scholar]
- 62.Davis M, Portmann B, Searle M, Wright R, Williams R. Histological evidence of carcinoma in a hepatic tumour associated with oral contraceptives. Br Med J. 1975;4:496–8. doi: 10.1136/bmj.4.5995.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gyorffy EJ, Bredfeldt JE, Black WC. Transformation of hepatic cell adenoma to hepatocellular carcinoma due to oral contraceptive use. Ann Int Med. 1989;110:489–90. doi: 10.7326/0003-4819-110-6-489. [DOI] [PubMed] [Google Scholar]
- 64.Perret AG, Mosnier JF, Porcheron J, Cuilleron M, Berthoux P, Boucheron S, Audigier JC. Role of oral contraceptives in the growth of a multilobular adenoma associated with a hepatocellular carcinoma in a young woman. J Hepatol. 1996;25:976–9. doi: 10.1016/s0168-8278(96)80305-9. [DOI] [PubMed] [Google Scholar]
- 65.Ye MQ, Suriawinata A, Haim MB, Parsons R, Schwartz ME. A 42 year old woman with liver masses and long term use of oral contraceptives. Semin Liv Dis. 1999;19(3):339–44. doi: 10.1055/s-2007-1007123. [DOI] [PubMed] [Google Scholar]
- 66.Tannapfel A, Wittekind C. Präneoplasien der Leber. Pathologe. 2001;22:399–406. doi: 10.1007/s002920100493. [DOI] [PubMed] [Google Scholar]
- 67.[Anonymous]. Terminology of nodular hepatocellular lesions–International working party. Hepatol. 1995;22(3):983–93. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 68.Weil R 3rd, Koep LJ, Starzl TE. Liver resection for hepatic adenoma. Arch Surg. 1979;114(2):178–80. doi: 10.1001/archsurg.1979.01370260068010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF 2nd. Hepatic adenoma and focal nodular hyper-plasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84(5):994–1002. [PubMed] [Google Scholar]
- 70.Belghiti J, Pateron D, Panis Y, Vilgrain V, Flejou JF, Benhamou JP, Fekete F. Resection of presumed benign liver tumours. Brit J Surg. 1993;80(3):380–3. doi: 10.1002/bjs.1800800340. [DOI] [PubMed] [Google Scholar]
- 71.Nagorney DM. Benign hepatic tumors: focal nodular hyperplasia and hepatocellular adenoma. World J Surg. 1995;19(1):13–8. doi: 10.1007/BF00316973. [DOI] [PubMed] [Google Scholar]
- 72.De Carlis L, Pirotta V, Rondinara GF, Sansalone CV, Colella G, Maione G, Slim AO, et al. Hepatic adenoma and focal nodular hyperplasia: diagnosis and criteria for treatment. Liv Transpl Surg. 1997;3(2):160–5. doi: 10.1002/lt.500030209. [DOI] [PubMed] [Google Scholar]
- 73.Ichikawa T, Federle MP, Grazioli L, Nalesnik M. Hepatocellular adenoma: multiphasic CT and histopathologic findings in 25 patients. Radiology. 2000;214(3):861–8. doi: 10.1148/radiology.214.3.r00mr28861. [DOI] [PubMed] [Google Scholar]
- 74.Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D, Badalamenti S, et al. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67(2):173–8. [PubMed] [Google Scholar]
- 75.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, et al. Management of 155 patients with benign liver tumours. Brit J Surg. 2001;88(6):808–13. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 76.Tao LC. Are oral contraceptive-associated liver cell adenomas premalignant? Acta cytol. 1992;36(3):338–44. [PubMed] [Google Scholar]
- 77.Loeb KR, Loeb LA. Significance of multiple mutations in cancer. Carcinogenesis. 2000;21(3):379–85. doi: 10.1093/carcin/21.3.379. [DOI] [PubMed] [Google Scholar]
- 78.Brunt EM, Swanson PE. Immunoreactivity for c-erbB-2 oncopeptide in benign and malignant diseases of the liver. Am J Clin Path. 1992;97(5 Suppl 1):S53–61. [PubMed] [Google Scholar]
- 79.Ding SF, Jalleh RP, Wood CB, Bowles L, Delhanty JD, Dooley J, Habib NA. Different DNA changes in primary and recurrent hepatocellular carcinoma. Gut. 1992;33(10):1433–5. doi: 10.1136/gut.33.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nasarek A, Werner M, Nolte M, Klempnauer J, Georgii A. Trisomy 1 and 8 occur frequently in hepatocellular carcinoma but not in liver cell adenoma and focal nodular hyperplasia. A fluorescence in situ hybridization study. Virchows Arch A Pathol Anat Histol. 1995;427(4):373–8. doi: 10.1007/BF00199385. [DOI] [PubMed] [Google Scholar]
- 81.Gaffey MJ, Iezzoni JC, Weiss LM. Clonal analysis of focal nodular hyperplasia of the liver. Am J Path. 1996;148(4):1089–96. [PMC free article] [PubMed] [Google Scholar]
- 82.Paradis V, Laurent A, Flejou JF, Vidaud M, Bedossa P. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology. 1997;26(4):891–5. doi: 10.1002/hep.510260414. [DOI] [PubMed] [Google Scholar]
- 83.Strassburg CP, Oldhafer K, Manns MP, Tukey RH. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol. 1997;52(2):212–20. doi: 10.1124/mol.52.2.212. [DOI] [PubMed] [Google Scholar]
- 84.D'Errico A, Deleonardi G, Fiorentino M, Scoazec JY, Grigioni WF. Diagnostic implications of albumin messenger RNA detection and cytokeratin pattern in benign hepatic lesions and biliary cystadenocarcinoma. Diagn Mol Path. 1998;7(6):289–94. doi: 10.1097/00019606-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 85.de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188(2):201–6. doi: 10.1002/(SICI)1096-9896(199906)188:2<201::AID-PATH339>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 86.Tannapfel A, Busse C, Geißler F, Wizigmann H, Hauss J, Wittekind C. INK4a-ARF alterations in liver cell adenoma. Gut. 2002;51(2):253–8. doi: 10.1136/gut.51.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takayasu H, Motoi T, Kanamori Y, Kitano Y, Nakanishi H, Tange T, Nakagawara A, Hashizume K. Two case reports of childhood liver cell adenomas harboring (-catenin abnormalities. Hum Pathol. 2002;33(8):852–5. doi: 10.1053/hupa.2002.125771. [DOI] [PubMed] [Google Scholar]
- 88.Torbenson M, Lee HJ, Choti M, Gage W, Abraham S, Montgomery E, Boitnott J, Wu TT. Hepatic adenomas: Analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol. 2002;15(3):189–96. doi: 10.1038/modpathol.3880514. [DOI] [PubMed] [Google Scholar]
- 89.Chen YJ, Chen PJ, Lee MC, Yeh SH, Hsu MT, Lin CH. Chromosomal analysis of hepatic adenoma and focal nodular hyperplasia by comparative genomic hybridization. Genes chromosomes cancer. 2002;35:138–43. doi: 10.1002/gcc.10103. [DOI] [PubMed] [Google Scholar]
- 90.Andersen PH, Packer JT. Hepatic adenoma—observations after estrogen withdrawal. Arch Surg. 1976;111:898–900. doi: 10.1001/archsurg.1976.01360260066018. [DOI] [PubMed] [Google Scholar]
- 91.Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello C, Giacomoni A, Osio C, et al. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33(3):234–6. doi: 10.1097/00004836-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 92.Steinbrecher UP, Lisbona R, Huang SN, Mishkin S. Complete regression of hepatocellular adenoma after withdrawal of oral contraceptives. Dig Dis Sci. 1981;26(11):1045–50. doi: 10.1007/BF01314771. [DOI] [PubMed] [Google Scholar]
- 93.Bühler H, Pirovino M, Akovbiantz A, Altorfer J, Weitzel M, Maranta E, Schmid M. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82:775–82. [PubMed] [Google Scholar]
- 94.Edmondson HA, Reynolds TB, Henderson B, Benton B. Regression of liver cell adenomas associated with oral contraceptives. Ann Int Med. 1977;86:180–2. doi: 10.7326/0003-4819-86-2-180. [DOI] [PubMed] [Google Scholar]
- 95.Ramseur WL, Cooper MR. Asymptomatic liver cell adenomas: another case of resolution after discontinuation of oral contraceptive use. JAMA. 1978;239:1647–8. doi: 10.1001/jama.239.16.1647. [DOI] [PubMed] [Google Scholar]
- 96.Heeringa B, Armando S. Bleeding hepatic adenoma: expectant treatment to limit the extent of liver resection. Am Surg. 2001;67(10):927–9. [PubMed] [Google Scholar]
- 97.Kay S. Nine year follow-up of a case of benign liver cell adenoma related to oral contraceptives. Cancer. 1977;40:1759–60. doi: 10.1002/1097-0142(197710)40:4<1759::aid-cncr2820400453>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 98.Mariani AF, Livingstone AS, Pereiras RV., Jr Progressive enlargement of a hepatic cell adenoma. Gastroenterology. 1979;77:1319–25. [PubMed] [Google Scholar]
- 99.Marks WH, Thompson N, Appleman H. Failure of hepatic adenomas to regress after discontinuance of oral contraceptives. Ann Surg. 1988;208(2):190–5. doi: 10.1097/00000658-198808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen C, Lawson D, DeRose PB. Sex and androgenic steroid receptor expression in hepatic adenomas. Hum Path. 1998;29(12):1428–32. doi: 10.1016/s0046-8177(98)90011-9. [DOI] [PubMed] [Google Scholar]
- 101.Torbenson M, Lee JH, Choti M, Gage W, Abraham SC, Montgomery E, Boitnott J, et al. Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol. 2002;15(3):189–96. doi: 10.1038/modpathol.3880514. [DOI] [PubMed] [Google Scholar]
- 102.Ribeiro A, Burgart LJ, Nagorney DM, Gores GJ. Management of liver adenomatosis: results with a conservative surgical approach. Liv Transpl Surg. 1998;4(5):388–98. doi: 10.1002/lt.500040505. [DOI] [PubMed] [Google Scholar]
- 103.Bertrand G, Saint-Andre JP, Simard C, Pillet J, Sulzer J. Letter: Nodular liver hyperplasia after oral contraception 2 new cases. Nouv Presse Med. 1975;4(31):2276. [PubMed] [Google Scholar]
- 104.Leborgne J, Lehur PA, Horeau JM, Dupas B, Bourcheix LM, Petiot JM, Cloarec D, et al. Therapeutic problems caused by rupture of large hepatic adenoma with central location. Apropos of 3 cases. Chirurgie. 1990;116(4–5):454–60. [PubMed] [Google Scholar]
- 105.Khan SS, Fink M, King S. Case report: liver adenomatosis presenting as multiple calcified masses. Clin Radiol. 1992;45(3):206–7. doi: 10.1016/s0009-9260(05)80645-2. [DOI] [PubMed] [Google Scholar]
- 106.Brander WL, Vosnides G, Ogg CS, West IE. Multiple hepatocellular tumours in a patient treated with oral contraceptives. Virchows Arch A Pathol Anat Histol. 1976;370(1):69–76. doi: 10.1007/BF00427311. [DOI] [PubMed] [Google Scholar]
- 107.Dereuri O, Guilhoi JJ. Multifocal hepatocellular carcinoma presenting as prurigo: two cases. Br J Dermat. 2000;143:1319–59. doi: 10.1046/j.1365-2133.2000.03919.x. [DOI] [PubMed] [Google Scholar]
- 108.Yunta PJ, Moya A, San-Juan F, Lopez-Andujar R, De Juan M, Orbis F, Mir J. A new case of hepatic adenomatosis treated with orthotopic liver transplantation. Ann Chir. 2001;126(7):672–4. doi: 10.1016/s0003-3944(01)00577-6. [DOI] [PubMed] [Google Scholar]
- 109.Mueller J, Keeffe EB, Esquivel CO. Liver transplantation for treatment of giant hepatocellular adenomas. Liv Transpl Surg. 1995;1(2):99–102. doi: 10.1002/lt.500010205. [DOI] [PubMed] [Google Scholar]
- 110.Pichlmayr R, Weimann A, Ringe B. Indications for liver transplantation in hepatobiliary malignancy. Hepatology. 1994;20:33–40S. doi: 10.1016/0270-9139(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 111.Adam R, Cailliez V, Majno P, Karam V, McKaster P, Calne RY, O'Grady J, et al. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356:621–7. doi: 10.1016/s0140-6736(00)02603-9. [DOI] [PubMed] [Google Scholar]
- 112.Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EGE, Klompmaker IJ, Slooff MJH, et al. Increased cancer risk after liver transplantation: A population-based study. J Hepatol. 2001;34(1):84–91. doi: 10.1016/s0168-8278(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 113.Cohen AJ, Stegall MD, Rosen CB, Wiesner RH, Leung N, Kremers WK, Zein NN. Chronic renal dysfunction late after liver transplantation. Liver Transpl. 2002;8(10):916–21. doi: 10.1053/jlts.2002.35668. [DOI] [PubMed] [Google Scholar]
- 114.Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl. 2001;7(1):S22–S26. doi: 10.1053/jlts.2001.28511. [DOI] [PubMed] [Google Scholar]
- 115.Testa G, Malago M, Nadalin S, Paul A, Frilling A, Broelsch CE. Complications and outcomes in adult living donor liver transplantation. Curr Opin Org Transpl. 2001;6(4):367–70. [Google Scholar]
- 116.Ramji A, Yoshida EM, Bain VG, Kneteman NM, Scudamore CH, Ma MM, Steinbrecher UP, et al. Late acute rejection after liver transplantation: The Western Canada experience. Liver Transpl. 2002;8(10):945–51. doi: 10.1053/jlts.2002.34969. [DOI] [PubMed] [Google Scholar]
- 117.Neumann UP, Langrehr JM, Neuhaus P. Chronic rejection after human liver transplantation. Graft. 2002;5(2):102–7. [Google Scholar]
- 118.Penna C. Femme de 57 ans qui presente des ‘nodules’ du foie. J Chir. 2002;139:40–5. [PubMed] [Google Scholar]
- 119.Tepetes K, Selby R, Webb R, Madariaga JR, Iwatsuki S, Starzl TE. Orthotopic liver transplantation for benign hepatic neoplasms. Arch Surg. 1995;130:153–6. doi: 10.1001/archsurg.1995.01430020043005. [DOI] [PubMed] [Google Scholar]
- 120.Chiappa A, Zbar A, Audisio R, Di Palo S, Bertani E, Staudacher C. Ruptured hepatic adenoma in liver adenomatosis– A case report of emergency surgical management. Hepato- gastroenterology. 1999;46:1942–3. [PubMed] [Google Scholar]