Abstract
Background. Hepatic resection for malignancies or symptomatic benign liver lesions remains the standard of treatment. Historically, the principal cause of mortality during liver resection was intraoperative bleeding. Advances in surgical and anesthetic techniques, along with application of new technologies, have decreased blood loss and dramatically improved the outcomes for major liver surgery.
Methods. The purpose of this prospective study was to determine the utility of a saline-cooled radiofrequency coagulation device (TissueLink Medical, Inc.) for hepatic resection. Intraoperative bleeding, blood transfusion, postoperative bile leak, and other complications were noted.
Results. The results are described for 170 patients undergoing hepatic resection over a three-year period. There were no intraoperative or postoperative deaths. Six patients in the series received blood transfusions for a transfusion rate of 3.5%. Four patients experienced a transient postoperative bile leak. Three of the four closed spontaneously prior to discharge home, and the fourth closed promptly after ERCP. There were no episodes of postoperative hemorrhage, hepatic failure, liver abscess, or reoperation.
Conclusions. The saline-cooled radiofrequency coagulation device is very effective in achieving intraoperative hemostasis and facilitates liver parenchymal transection during hepatic resection.
Keywords: Blood conservation, colon cancer, HCC, hepatic resection, liver cancer, liver resection, radiofrequency coagulation
Introduction
Hepatic resections for tumors or symptomatic benign liver lesions have been performed for more than 50 years 1. Traditionally, the principal cause of morbidity and mortality in hepatic resection was directly related to intraoperative bleeding 2,3,4,5. There are many anatomical challenges that make liver surgery complex. The liver has a dual blood supply consisting of the hepatic artery and portal vein, and receives a significant proportion of cardiac output. Most high volume medical centers with specialized hepatobiliary units report median blood loss of 800–1800 ml for major liver resections, with operative transfusion rates of 15%–35% 6,7,8,9,10,11. Large resection series in cirrhotic patients indicate even higher blood loss and transfusion rates 12,13,14. Peri operative blood transfusion is a risk factor for poor outcome after liver resection, and blood conservation methods should be used to avoid transfusion 15.
Most of the blood loss during hepatic resection is encountered during transection of the liver parenchyma. With anatomical resection, this blood loss can be minimized with prior control of the inflow pedicle and division of ipsilateral hepatic vein. Non-anatomical resections and division of steatotic or cirrhotic livers are notoriously difficult due to bleeding from the parenchyma. A better understanding of hepatic anatomy, advances in surgical technique, and refinements in anesthetic management have dramatically improved the outcomes of major hepatic resection in the last decade 16,17,18,19,20. This has also led to a widespread acceptance of live donor hepatectomies for liver transplantation 21,22.
Various methods and techniques have been described to divide the liver parenchyma, but none are ideal. Desire to minimize blood loss, and ability to control blood vessels and bile ducts encountered during parenchymal division, have to be balanced against lateral damage to remaining tissue and vital structures. In recent times, further technological advances have resulted from an explosion in minimally invasive technology. Many of these innovative instruments have been applied in open surgical procedures to reduce blood loss. Liver surgeons today have a wide array of electrosurgical devices, staplers, and hemostatic agents to choose from for operative hepatic surgery. In this report, we describe our experience with 170 hepatic resections using a saline-cooled radio-frequency coagulation device.
Methods
Patient and operative features
One hundred seventy open liver resection procedures were performed at the University of Pittsburgh Medical Center/Starzl Transplant Institute from April 2001 to May 2004. Of the series, 55% were male and 45% female. The mean patient age was 62 years old. The indications for liver resection are shown in Table I. Malignancy was the indication for hepatic resection in 121/170 patients (71%), and 49/170 (29%) were for symptomatic benign liver lesions. The most common indication for malignancy was resection of colorectal cancer metastases, followed by resection for HCC. Cirrhosis was present in 10/170 patients (6%), and all of these patients were considered Child's A. The operative procedures performed are shown in Table II. Fifty-three percent of the cases consisted of formal hepatectomies (lobectomy, trisegmentectomy, or left lateral segmentectomy). An additional wedge resection of a second tumor was performed in 29 patients. Open radiofrequency ablation of a liver tumor was combined with hepatic resection in 46 patients. An implantable hepatic artery infusion pump was also placed following resection for colorectal metastases in 16 patients.
Table I. Indication for hepatic resection.
| Liver lesion | # Patients | % |
|---|---|---|
| Colon/rectal cancer metastasis | 70 | 41 |
| Hepatocellular carcinoma (HCC) | 13 | 8 |
| Cholangiocarcinoma | 8 | 5 |
| Gallbladder cancer | 10 | 6 |
| Neuroendocrine tumor | 4 | 2 |
| Breast cancer metastasis | 6 | 3 |
| Sarcoma | 3 | 2 |
| Other metastatic cancer | 7 | 4 |
| Adenoma | 5 | 3 |
| Focal nodular hyperplasia (FNH) | 7 | 4 |
| Hemangioma | 18 | 11 |
| Cyst/polycystic liver | 12 | 7 |
| Abscess | 5 | 3 |
| Trauma | 2 | 1 |
| Total | 170 | 100 |
Table II. Type of hepatic resection performed.
| Operative procedure | # Cases | % |
|---|---|---|
| Right hepatic lobectomy | 49 | 29 |
| Left hepatic lobectomy | 16 | 9 |
| Left lateral segmentectomy | 20 | 12 |
| Trisegmentectomy/extended lobectomy | 5 | 3 |
| Partial lobectomy/wedge resection | 65 | 38 |
| Central liver resection | 9 | 5 |
| Caudate lobectomy | 1 | 1 |
| Cystectomy | 5 | 3 |
| Total | 170 | 100 |
Saline-cooled radiofrequency coagulation devices
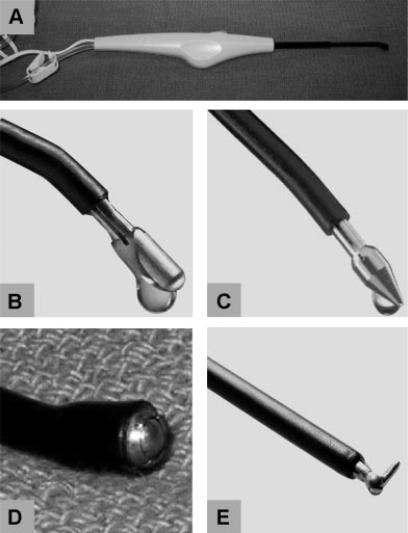
The saline-cooled radiofrequency-powered devices (TissueLink Medical, Inc, Dover, NH) utilized are shown in Figure 1. The original device is the Tissue-Link Floating Ball (Figure 1A and 1D). The device plugs into a standard operating room Bovie electro-surgical generator. The coagulation power is typically set between 90–100 W. A sterile liter of 0.9% saline is connected to the irrigation tubing and adjusted for a drip rate of 4–8 cc/minute. The device conducts radiofrequency energy from the generator to the electrode tip where continuous low-volume saline irrigation cools the contact interface with the hepatic tissue, keeping surface temperature 100–105°C. This avoids the eschar and char formation that occurs with Bovie electrocautery pencil. The thermal energy results in heat that denatures protein in the vessel wall and shrinks collagen, thereby sealing blood vessels. Subsequent design modifications of the Floating Ball allow for combined dissection and sealing with an exposed blunt or sharp (cone-head) distal tip (Figure 1B, DS3.0, and Figure 1C, DS3.5c). Another modification is the SH Hook (Figure 1E) that can be used to facilitate laparoscopic liver resection.
Figure 1. .
Selection of TissueLink coagulation devices. The device is a hand-held, saline-cooled radiofrequency-powered electrode that plugs into a standard electrosurgical generator (A). Shown are different tip configurations consisting of blunt DS3.0 (B), sharp DS3.5c (C), Floating Ball (D), and SH Hook (E).
Surgical technique
If a formal lobectomy is to be performed, then the ipsilateral hepatic artery and portal vein branch are ligated in the hilum. The liver is elevated from the inferior vena cava, and short hepatic veins are secured, thereby exposing the juncture of the right and middle/ left hepatic veins with the IVC. The right hepatic vein (for right lobectomy) or middle/left hepatic veins (for left lobectomy) are divided with endoGIA vascular stapler under direct vision 23. Intraoperative liver ultrasound is routinely performed to identify extent of tumor and help delineate the optimal resection plane. If a non-anatomic partial lobectomy or large wedge resection is to be performed, then the operation proceeds directly with the parenchymal transection phase.
The liver capsule is scored with electrocautery for a few mm of depth. This is important to prevent gas/ steam build-up under Glisson's capsule and avoid a large popping/explosion noise 24. The liver tissue is then ‘precoagulated’ by holding constant pressure with the radiofrequency coagulation device and stroking along the transection line in a ‘painting manner’. Small microbubbles of boiling saline are observed and the liver tissue will actually turn a whitish-yellow color (rather than black eschar observed with dry cautery). The device works well at a 60° angle, and can be gently rotated/swirled within the fingers to avoid adhesion. The first assistant uses a suction device adjacent to the contact site along the line of resection. If there is too much or too little saline, then proper function is impeded. The depth of thermal coagulation is proportional to the power setting of the generator and duration of contact with the liver parenchyma. In general, a 5 mm depth of thermal coagulation is obtained. Once adequate coagulation is achieved, the hepatic parenchyma can be divided with Bovie electrocautery or scissors. If the cone-tipped DS3.5c is being used, then the liver can actually be divided by short tension-controlled downward sweeping strokes of the sharp tip. If bleeding is encountered while progressing through the resection zone, hemostasis can, be achieved by rolling over this area with the side of the device.
When crossing blood vessels are encountered, they are skeletonized by dissection on each side and then coagulated by gentle, direct contact above and below the blood vessel for a few seconds. The saline-cooled radiofrequency coagulation devices function as a ‘wet electrode’ and can readily seal blood vessels 3–6 mm in size. Larger vessels are secured with clips, suture ligatures, or vascular staplers. The TissueLink devices also work quite well in combination with an ultrasonic or hydrojet dissector 25. These dissectors aspirate the hepatic parenchyma and expose the crossing blood vessels that can rapidly be coagulated and sealed by the radiofrequency coagulation devices. In the 170 cases reported in the current series, approximately one-third utilized the Floating Ball, one-third the DS3.0, and one-third the DS3.5c.
Evolution of transection technique
In the last 50 cases of major hepatic resection, our preferred parenchymal transection technique has evolved to begin with standard cautery to score the surface (capsule) of the liver along the plane of division determined by intraoperative ultrasound. This is followed by precoagulation and dissection with the TissueLink sharp (cone head) tip to a depth of 3–4 cm in the parenchyma or until dominant crossing middle hepatic vein branches are encountered. Subsequently, inflow occlusion is achieved with a Potts-looped wide vessel loop around the hepatoduodenal ligament secured with a blunt right-angle clamp. The remainder of the liver parenchymal transection is accomplished with repetitive application of a crushing endoGIA vascular stapler (45 mm straight blue cartridge) passed after tunneling with a large Kelly clamp. Hemostasis of any oozing parenchyma is then completed with the TissueLink device. Argon beam coagulator is not used on the cut edge of the liver. For formal right hemi-hepatectomy, total inflow occlusion time has ranged from 4 minutes to 18 minutes (mean 10 minutes) to complete the lobectomy. Advantage of the combined TissueLink and staple technique is the rapid speed by which the entire right lobe parenchymal ‘slice’ can be performed. Disadvantage is the cost of these staplers, and at times the transected liver surface may not be as flat as achieved with other methods, necessitating the placement of a few figure-of-eight hemostatic sutures.
Results
Post-op morbidity
Hepatic resection was successfully performed in 170 patients over a three-year period. There were no intraoperative or postoperative deaths in the series. There were no episodes of postoperative hemorrhage, liver failure, or reoperation. Postoperative morbidity included a few patients with ileus requiring transient replacement of nasogastric tube. No abdominal or hepatic abscesses were noted related to the coagulation zone at the remnant liver edge. One patient who underwent central liver resection for gallbladder cancer experienced a pulmonary embolus two weeks post-operatively and was readmitted for anticoagulation. Mean and median length of stay was 6.5 and 6.0 days, respectively.
Blood transfusions
Blood transfusions were given in six patients, for an overall transfusion rate of 3.5% (6/170). Five patients received the packed red blood cells (pRBC) in the operating room, and one patient received 2 units of pRBC in the recovery room when her postoperative hematocrit was 17.3%. In those patients requiring blood transfusion, the number of units of pRBC transfused ranged from 1–3 units, with a mean of 2.0 units. Average estimated blood loss (EBL) was 100 cc for the liver parenchymal transection phase. The range of EBL was 25–500 cc. Since recorded EBL is some-what subjective and often differs in estimate between the surgeon, the anesthesiologist, and the circulating nurse, we also calculated ΔHct (preoperative hematocrit-nadir postoperative hematocrit) to provide an objective assessment of changes in blood count. The ΔHct reflects actual blood loss as well as hemodilution by perioperative fluid replacement. The mean ΔHct for the 170 patients was 7% (range 0%–24%).
Bile leaks
A closed-suction bulb drain was routinely placed next to the cut-edge of the liver in ∼95% of cases to monitor for postoperative bleeding or bile leak. Patients undergoing small wedge resections were closed without a drain. Bile leaks were observed in 4/170 patients for an overall leak rate of 2.3%. In three of the patients, the bile leak sealed spontaneously on post-operative days #3, 4, and 4. The fourth patient required an ERCP that resulted in prompt closure. There were no reoperations or infections related to the bile leaks. This low bile leak rate may be attributed in part to the practice of the surgical team routinely performing cholecystectomy and completion intraoperative cholangiogram through the cystic duct stump to assess for bile leak from the cut edge or divided hepatic duct stump. Any detected bile leaks were oversewn with prolene suture.
Discussion
In the current study, saline-cooled radiofrequency coagulation was used to achieve liver hemostasis in 170 patients undergoing hepatic resection. The technique consists of precoagulation of liver parenchyma along the line of resection with a hand-held device that plugs into a conventional Bovie generator, followed by tissue division using scissors or Bovie cautery. The newer generation electrodes have tip configurations that allow for simultaneous dissection and coagulation. Six patients in the series received blood transfusions for a transfusion rate of 3.5%. Four patients experienced a transient postoperative bile leak, and three of the four closed spontaneously prior to discharge home. The devices appear safe as there were no instances of hepatic failure, postoperative hemorrhage, or liver abscess. Further, the low rate of blood transfusion is significantly less than our own previous reports 3,4,5,6, or the 15%–33% transfusion rate reported in most large hepatic resection series 7,8,9,10,11.
For several years before adopting this technique, the parenchymal transection was accomplished by passing a large right angle clamp through the liver tissue that was secured with crushing ties. It was our experience that the incidence of post-operative bile leaks, and need for blood transfusion was greater with this earlier approach. Since this was not a prospective, randomized study, we are cautious in not directly comparing the results of the two techniques. The cost of the TissueLink device is approximately $875 per device. Prior to use of the TissueLink device, a harmonic scalpel, as well as application of a fibrin glue sealant to the cut edge of the liver, was often utilized. Although a formal cost-analysis has not been performed, we believe the savings in avoiding the use of fibrin glue and other hemostatic devices balance the cost of the TissueLink device.
This study is the largest reported series using saline-cooled radiofrequency coagulation for hepatic resection. Sturgeon et al. described their early experience with the Floating Ball device in seven patients undergoing hepatic resection for HCC or colorectal cancer metastases 26. There were no blood transfusions, postoperative bile leaks, or hemorrhages. Sakamoto et al. recently reported on 16 hepatic resections where the Floating Ball was used in combination with a bipolar LigaSure diathermy device 27. In this study, there was significantly less blood loss compared to well-matched historical controls where the liver was divided with conventional crush clamp method during inflow occlusion. It has also been reported for use in liver resection combined with ultrasonic surgical aspirator 28.
Multiple surgical techniques have been described to control bleeding during hepatic resection. This includes extra-hepatic ligation of the hilar structures, intra-hepatic pedicle ligation along Glisson's capsule, Pringle maneuver, half-Pringle maneuver, and total vascular exclusion 29,30,31,32,33,34. The advent of electro-surgical devices revolutionized surgery, and most operating rooms worldwide utilize a conventional Bovie-type electrosurgical generator, especially for hepatic surgery. The Bovie electrocautery can be used during hepatic surgery to score the liver capsule and effectively seal off small blood vessels (1–2 mm). However, to achieve hemostasis for larger blood vessels in the liver parenchyma, higher power is required resulting in excessive heat. The temperature often exceeds 350°C, causing significant lateral damage, charring, and eschar formation. Eschar usually sticks to the electrode and has a tendency to separate from the liver parenchyma as the electrode is moved. Charring makes it difficult to distinguish between parenchyma, vascular, and biliary structures. It is difficult to divide the liver parenchyma with conventional Bovie electrocautery alone without risking major blood loss.
The TissueLink devices combine radiofrequency electrical energy with low irrigation of saline to conduct energy and provide a cooling effect to the tissue. This is capable of sealing structures 3–6 mm in diameter without producing high temperature or excessive charring and eschar. Structures more than 6 mm in diameter should be divided in conventional manner with clips or ties. Constant suction is required to clear the saline used for irrigation. The first generation TissueLink device utilized a floating ball tip that ‘precoagulated’ liver tissue which is then divided with scissors 26 or Bovie cautery. One common criticism is the slow pace of liver resection with this device. This was improved with later generation devices consisting of a blunt or sharp (cone head) tip that allow for dissection and sealing. These devices can also be used in combination with ultrasonic dissector or hydrojet to shorten parenchymal transection time. Properly used, these devices can result in near ‘bloodless’ liver surgery. Similar to most new technologies, there is a learning curve to optimize use of the instrument, and most surgeons are comfortable after 5–6 procedures.
Recently, application of an internally cooled tip radiofrequency (RF) probe to precoagulate liver tissue along the planned transection line has also been described 35,36,37. In these studies, a single RF electrode is inserted sequentially into the liver parenchyma under ultrasound guidance to achieve a 1-cm zone of necrosis along the planned resection line. The hepatic resection is then performed with a scalpel 35,36,37. One disadvantage of this technique compared to the TissueLink devices is that a specialty generator is required. Another disadvantage is the need for multiple insertions of the device deep into the parenchyma, whereas the TissueLink devices allow for simultaneous dissection and coagulation under direct vision. Others have reported on the use of bipolar devices, ultrasonic shears, and fibrin sealants to help achieve hemostasis during hepatic surgery 38,39,40,41. Saline-cooled radio-frequency coagulation has also been used in the kidney 42,43,44. In these cases, the device was used to facilitate dissection and achieve hemostasis during partial nephrectomy for renal cell carcinoma.
In summary, we have shown that application of saline-cooled radiofrequency coagulation is very effective in achieving hemostasis during hepatic surgery. Use of the device alone or concurrent with other techniques results in a very low rate of blood transfusion or postoperative bile leak.
Acknowledgements
Dr Geller is a consultant to TissueLink Medical, Inc. This manuscript was presented in part at the 6th World Congress of the International Hepato-Pancreato-Biliary Association (IHPBA), 5 June 2004.
References
- 1.Fortner JG, Blumgart LH. A historic perspective of liver surgery for tumors at the end of the millennium. J Am Coll Surg. 2001;193:210–22. doi: 10.1016/s1072-7515(01)00910-3. [DOI] [PubMed] [Google Scholar]
- 2.Fortner JG, Kim DK, Maclean BJ, Barrett MK, Iwatsuki S, Turnbull AD, Howland WS, Beattie EJ., Jr Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188:363–71. doi: 10.1097/00000658-197809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Iwatsuki S, Shaw BW, Jr, Waterman PM, Van Thiel D, Diliz HS, Dekker A, Bron KM. Left hepatic trisegmen-tectomy. Surg Gynecol Obstet. 1982;155:21–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Iwatsuki S, Esquivel CO, Gordon RD, Starzl TE. Liver resection for metastatic colorectal cancer. Surgery. 1986;100:804–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208:421–34. doi: 10.1097/00000658-198810000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwatsuki S, Starzl TE. Experience with resection of primary hepatic malignancy. Surg Clin North Am. 1989;69:315–22. doi: 10.1016/s0039-6109(16)44788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 8.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 9.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez JA, Arslan V, Fischer ME, Wuest W, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: Blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–5. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 11.Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526–9. doi: 10.1002/bjs.1800831110. [DOI] [PubMed] [Google Scholar]
- 12.Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. doi: 10.1001/archsurg.1995.01430020088017. [DOI] [PubMed] [Google Scholar]
- 13.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–11. doi: 10.1097/00000658-200211000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capussotti L, Muratore A, Massucco P, Ferrero A, Polastri R, Bouzari H. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl. 2004;10:S64–8. doi: 10.1002/lt.20035. [DOI] [PubMed] [Google Scholar]
- 15.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–9. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariette D, Smadja C, Naveau S, Borgonovo G, Vons C, Franco D. Preoperative predictors of blood transfusion in liver resection for tumor. Am J Surg. 1997;173:275–9. doi: 10.1016/S0002-9610(96)00400-X. [DOI] [PubMed] [Google Scholar]
- 17.Buell JF, Koffron A, Yoshida A, Hanaway M, Lo A, Layman R, Cronin DC, Posner MC, Millis M. Is any method of vascular control superior in hepatic resection of metastatic cancers? Longmire clamping, pringle maneuver, and total vascular isolation. Arch Surg. 2001;136:569–75. doi: 10.1001/archsurg.136.5.569. [DOI] [PubMed] [Google Scholar]
- 18.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, Frena A, Morganti M, Ercolani G, Masetti M, et al. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–10. doi: 10.1002/bjs.1800820833. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson GG, Corbel L, Campion JP, Launois B. Major liver resection without a blood transfusion: is it a realistic objective? Surgery. 1992;112:32–6. [PubMed] [Google Scholar]
- 21.Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Posner MP. Right lobe living donor liver transplantation. Transplantation. 1999;68:798–803 T. doi: 10.1097/00007890-199909270-00012. [DOI] [PubMed] [Google Scholar]
- 22.Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, Branchereau S, Chardot C, Gauthier F, Fagniez PL, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359:392–6. doi: 10.1016/S0140-6736(02)07598-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang WX, Fan ST. Use of the Endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003;26:193–6. doi: 10.1016/S1015-9584(09)60301-8. [DOI] [PubMed] [Google Scholar]
- 24.Topp SA, McClurken M, Lipson D, Upadhya GA, Ritter JH, Linehan D, Strasberg SM. Saline-linked surface radio-frequency ablation: factors affecting steam popping and depth of injury in the pig liver. Ann Surg. 2004;239:518–27. doi: 10.1097/01.sla.0000118927.83650.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rau HG, Buttler ER, Baretton G, Schardey HM, Schildberg FW. Jet-cutting supported by high frequency current: New technique for hepatic surgery. World J Surg. 1997;21:254–60. doi: 10.1007/s002689900225. [DOI] [PubMed] [Google Scholar]
- 26.Sturgeon C, Helton WS, Lamba A, Chejfec G, Espat NJ. Early experience employing a linear hepatic parenchyma coagulation device. J Hepatobiliary Pancreat Surg. 2003;10:81–6. doi: 10.1007/s10534-002-0823-7. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, Muto T, Makuuchi M. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166–72. doi: 10.1007/s00268-003-7167-5. [DOI] [PubMed] [Google Scholar]
- 28.Gruttadauria S, Doria C, Vitale C, Mandala L, Magnone M, Fung JJ, Marino IR. New technique in hepatic parenchymal transection for living related liver donor and liver neoplasms. HPB. 2004;6:106–9. doi: 10.1080/13651820410025093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launois B, Jamieson GG. The importance of Glisson's capsule and its sheaths in the intrahepatic approach to resection of the liver. SurgGynecol Obstet. 1992;174:7–10. [PubMed] [Google Scholar]
- 30.Figueras J, Lopez-Ben S, Llado L, Rafecas A, Torras J, Ramos E, Fabregat J, Jaurrieta E. Hilar dissection versus the ‘Glissonean’ approach and stapling of the pedicle for major hepatectomies: A prospective, randomized Trial. Ann Surg. 2003;238:111–9. doi: 10.1097/01.SLA.0000074981.02000.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horgan PG, Leen E. A simple technique for vascular control during hepatectomy: the half Pringle. Am J Surg. 2001;182:265–7. doi: 10.1016/s0002-9610(01)00697-3. [DOI] [PubMed] [Google Scholar]
- 32.Huguet C, Addario-Chieco P, Gavelli A, Arrigo E, Harb J, Clement RR. Technique of hepatic vascular exclusion for extensive liver resection. Am J Surg. 1992;163:602–5. doi: 10.1016/0002-9610(92)90567-b. [DOI] [PubMed] [Google Scholar]
- 33.Emond JC, Kelley SD, Heffron TG, Nakagawa T, Roberts JP, Lim RC., Jr Surgical and anesthetic management of patients undergoing major hepatectomy using total vascular exclusion. Liver Transpl Surg. 1996;2:91–8. doi: 10.1002/lt.500020202. [DOI] [PubMed] [Google Scholar]
- 34.Hansen PD, Isla AM, Habib NA. Liver resection using total vascular exclusion, scalpel division of the liver parenchyma, and a simple compression technique for hemostasis and biliary control. J Gastrointest Surg. 1999;3:537–42. doi: 10.1016/s1091-255x(99)80109-7. [DOI] [PubMed] [Google Scholar]
- 35.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stella M, Percivale A, Pasqualini M, Profeti A, Gandolfo N, Serafini G, Pellicci R. Radiofrequency-assisted liver resection. J Gastrointest Surg. 2003;7:797–801. doi: 10.1016/s1091-255x(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 37.Zacharoulis D, Asopa V, Navarra G, Nicholls JP, Jensen SL, Habib NA. Hepatectomy using intraoperative ultrasound-guided radiofrequency ablation. Int Surg. 2003;88:80–2. [PubMed] [Google Scholar]
- 38.Strasberg SM, Drebin JA, Lineban D. Use of a bipolar vessel-sealing device for parenchymal transaction during liver surgery. J Gastrointest Surg. 2002;6:569–74. doi: 10.1016/s1091-255x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 39.Kokudo K, Kimura H, Yamamoto H, Seki M, Ohta H, Matsubara T, Takahashi T. Hepatic parenchymal transaction using ultrasonic coagulating shears: a preliminary report. J Hepatobiliary Pancreat Surg. 2000;7:295–8. doi: 10.1007/s005340070051. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Ahmad SA, Lowy AM, Buell JF, Pennington LJ, Soldano DA, James LE, Matthews JB, Hanto DW. Increased biliary fistulas after liver resection with the Harmonic Scalpel. Am Surg. 2003;69:815–9. [PubMed] [Google Scholar]
- 41.Busuttill RW. A comparison of antifibrinolytic agents used in hemostatic fibrin sealants. J Am Coll Surg. 2003;197:1021–8. doi: 10.1016/j.jamcollsurg.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Urena R, Mendez F, Woods M, Thomas R, Davis R. Laparoscopic partial nephrectomy of solid renal masses without hilar clamping using a monopolar radio frequency device. J Urol. 2004;171:1054–6. doi: 10.1097/01.ju.0000103927.75499.5d. [DOI] [PubMed] [Google Scholar]
- 43.Simon SD, Ferrigni RG, Novicki DE, Lamm DL, Swanson SS, Andrews PE. Mayo Clinic Scottsdale experience with laparoscopic nephron sparing surgery for renal tumors. J Urol. 2003;16:2059–62. doi: 10.1097/01.ju.0000058407.28232.38. [DOI] [PubMed] [Google Scholar]
- 44.Sundaram CP, Rehman J, Venkatesh R, Lee D, Rageb MM, Kibel A, Landman J. Hemostatic laparoscopic partial nephrectomy assisted by a water-cooled, high-density, monopolar device without renal vascular control. Urology. 2003;61:906–9. doi: 10.1016/s0090-4295(02)02550-5. [DOI] [PubMed] [Google Scholar]