Abstract
Background. Radiofrequency ablation (RFA) has been increasingly utilized for treatment of hepatocellular carcinoma (HCC). Long-term results of RFA, especially in comparison to surgical resection, have not been well described.
Methods. Eighty-seven patients with single nodule HCC underwent surgical resection (N=47) or RFA (N=40) during a 9-year period. RFA was performed for 36 unresectable disease and 4 surgical refusals. Each group was further divided based on tumor size for analysis; Group 1: resection, ≤5 cm (N=18), Group 2: RFA, ≤5 cm (N=26), Group 3: resection, >5 cm (N=29) and Group 4: RFA, >5 cm (N=14). Follow-up ranged from 2 to 72 months (median 16 months). Patients' characteristics, local recurrences and overall and disease-free survivals were compared.
Results. Patients who underwent RFA were older (69 versus 60, p=0.0006), had more advanced Child-Pugh class and TNM stage (p=0.0002 and p=0.016, respectively), and had smaller tumors (4.6 versus 7.4 cm, p=0.0032). Local recurrence rates were 2% for resection and 10% for RFA (p=0.12). These local and other recurrences were subsequently treated with multimodal therapies as indicated. The median overall and disease-free survivals were equivalent both between Groups 1 and 2 (49 versus 51 months, p=0.44, 36 versus 22 months, p=0.84), and Groups 3 and 4 (47 versus 463 months, p=0.94, 28 versus 20 months, p=0.67).
Discussion. Although the groups were not truly comparable, this retrospective study suggests that RFA may offer similar long-term results to surgical resection for single nodule HCC when combined with multimodal treatments.
Keywords: Hepatocellular carcinoma, liver transplant, long-term outcome, radiofrequency ablation, resection
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Although it is relatively uncommon in Western countries, its incidence is rising 1. Surgical resection and liver transplantation are considered as the only potentially curative treatments. The long-term results after surgical resection have improved with recent reported overall 5-year survival rates up to 50% 2. Perioperative complications have also been reduced with improvements in surgical and anesthetic techniques as well as perioperative patient management. The majority of HCC occurs in cirrhotic liver and resection rates are only 10%–37% even in tertiary referral centers 3. Liver transplantation, the only alternative curative treatment is also limited in its application due to donor shortage. Local ablative treatments for HCC include percutaneous ethanol injection, cryotherapy, laser, radio-frequency ablation (RFA) and others. RFA has been increasingly utilized because of its safety, versatility and applicability. Because RFA is a relatively new treatment modality, most reports in the literature are short-term. There have been several studies with promising long-term results 4,5; however, how RFA compares with surgical resection for HCC has not been well established. In order to truly compare RFA and surgical resection, randomized controlled studies are required. This would be difficult to perform because many believe surgical resection is the first-line treatment, while RFA is reserved for unresectable disease. We, therefore, retrospectively analyzed 87 patients with single nodule HCC who underwent either surgical resection or RFA at two tertiary referral community hospitals.
Methods
A total of 87 patients with single nodule HCC who underwent surgical treatments between January 1995 and August 2003 at St. Francis Medical Center and Kuakini Medical Center, Honolulu, HI, were retrospectively reviewed. Surgical decision making was based on history, physical examination, laboratory tests including complete blood counts, coagulation profile, liver functions, ultrasound (US) or triple-phase helical computed tomography (CT) findings. Hepatitis B and C profiles and serum alpha-fetoprotein (AFP) levels were obtained preoperatively. Surgical resection was considered in Child's A patients or early Child's B (Child-Pugh score of 7) patients without any evidence of ascites or encephalopathy. HCC was diagnosed pathologically by percutaneous biopsy or liver biopsy done at the time of surgery. During the study period, 47 patients underwent surgical resection. Forty patients underwent RFA, including 36 patients with unresectable lesions or poor hepatic reserve and 4 patients who refused surgical resection. To facilitate comparison among patients with equivalent tumor size, each treatment arm was further divided into two subgroups based on tumor size; Group 1: resection, ≤5 cm (N = 18), Group 2: RFA, ≤5 cm (N = 26), Group 3: resection, >5 cm (N=29) and Group 4: RFA, >5 cm (N=14). Patients with multiple tumors which were treated by resection or RFA and transplant candidates were not included in this study.
RFA was performed under ultrasonographic guidance, utilizing a generator providing 460 kHz alternating current and a cannula with retractable multi-pronged curved electrode-needles (RITA Medical System, Mountain View, California, or Radio-therapeutics Corporation, Mountain View, California), as previously reported 6,7. Most procedures were performed under general anesthesia in the operating room by percutaneous, laparoscopic or laparotomy approach. The average target temperature was set at 100°C to 110°C, and ablation was continued for 5–30 minutes depending on the desired ablation size (3–5 cm in diameter). The process was monitored by real-time ultrasound to ensure 1-cm margins. For large tumors, multiple overlapping ablations were performed.
During follow up after RFA, a triple-phase helical computed tomography was obtained one week post-operatively, and then at 3-month intervals for one year and at 4- to 6-month intervals thereafter. AFP was measured a few weeks postoperatively, and then at 2-to 4-month intervals. Similar follow-up was performed after surgical resection. Whenever AFP re-elevated, further imaging studies such as CT scan and gallium scan were performed. Local recurrence was defined as tumor recurrence at the treated site, whereas new tumor which appeared in different hepatic parenchyma was defined as new intrahepatic recurrence. For these recurrences, RFA treatment or re-resection was considered and performed as indicated. Other patients were considered for regional (transarterial chemo-embolization or hepatic artery infusion chemotherapy) or systemic chemotherapy. All patients were followed for 2–72 months (median 16 months).
Patients' characteristics including age, gender, ethnicity, liver function, etiology of cirrhosis, AFP, TNM stage (American Joint Committee on Cancer, 5th edition), Child-Pugh classification 8,9 were compared among groups. Local and new intrahepatic recurrence rates and overall and disease-free survivals were assessed.
Data were collected retrospectively and analyzed with SAS 8.02 for Windows (SAS Institute Inc., Cary, North Carolina) statistical software. Continuous variables were expressed as mean±standard deviation and significance between different groups was determined by chi-square test. Survival curves were evaluated by Kaplan-Meier's method and log-rank test was used to assess significance between groups. For all comparisons, significance was at 0.05 level.
Results
Patients who underwent resection were not completely comparable to those who underwent RFA. These demographics are summarized in Table I. There was no difference in the male to female ratio, ethnicity, AFP and risk factors of hepatitis C and alcohol. Patients who underwent RFA were significantly older and had more advanced TNM stage and worse underlying liver function as determined by Child-Pugh class.
Table I. Chararteristics of patients by treatment.
| Resection (N = 47) | RFA (N = 40) | p-value | |
|---|---|---|---|
| Age (years) | 60±12 | 69±10 | 0.0006 |
| Male/female | 29/18 | 19/21 | NS |
| Tumor size (cm) | 7.4±5.2 | 4.6±2.9 | 0.0032 |
| AFP (ng/mL) | 37,900±148,100 | 636±1724 | NS |
| Etiology of cirrhosis | |||
| HBV | 19 (40%) | 7 (18%) | 0.02 |
| HCV | 10 (21%) | 16 (40%) | NS |
| Alcoholic | 8 (17%) | 10 (25%) | NS |
| Child-Pugh | 0.0002 | ||
| A | 40 (85%) | 18 (45%) | |
| B | 7 (15%) | 18 (45%) | |
| C | 0 (10%) | 4 (10%) | |
| TNM Stage | 0.016 | ||
| 1 | 5 (11%) | 2 (5%) | |
| 2 | 37 (79%) | 28 (70%) | |
| 3 | 3 (6%) | 0 (0%) | |
| 4 | 2 (4%) | 10 (25%) | |
AFP: alpha-fetoprotein; HBV: hepatitis B virus; HCV: hepatitis C virus; NS: not significant.
Those who underwent resection had larger tumors and were more likely to have hepatitis B. When patients were divided into subgroups as shown in Table II, some of these differences were no longer noted. In patients with tumors of 5 cm or less (Groups 1 and 2), those who underwent RFA were older and a higher Child-Pugh score than those who underwent resection. The mean tumor size was comparable between Groups 1 and 2. In patients with tumors larger than 5 cm (Groups 3 and 4), those who underwent RFA were older, had smaller tumors and had more advanced Child-Pugh class and TNM stage.
Table II. Characteristics of patients by treatment and tumor size.
| Group 1 Resection ≤5 cm | Group 2 RFA ≤5 cm | p-value | Group 3 Resection >5 cm | Group 4 >RFA 5 cm | p-value | |
|---|---|---|---|---|---|---|
| Number of patients | 18 | 26 | 29 | 14 | ||
| Age (years) | 61±9 | 67±10 | 0.048 | 60±13 | 72±10 | 0.005 |
| Male/female | 12/6 | 10/16 | NS | 17/12 | 9/5 | NS |
| Tumor size (cm) | 2.9±1.1 | 3.3±0.8 | NS | 10.2±4.7 | 7.1±3.7 | 0.034 |
| AFP | 648±1677 | 700±1814 | NS | 62735±188455 | 517±1603 | NS |
| Etiology of cirrhosis | ||||||
| HBV | 6 (33%) | 4 (15%) | NS | 13 (45%) | 3 (21%) | NS |
| HCV | 7 (39%) | 15 (58%) | NS | 3 (10%) | 1 (7%) | NS |
| Alcoholic | 5 (28%) | 8 (31%) | NS | 3 (10%) | 2 (14%) | NS |
| Child-Pugh | 0.024 | 0.014 | ||||
| A | 14 (78%) | 10 (38%) | 26 (90%) | 8 (57%) | ||
| B | 4 (22%) | 12 (46%) | 3 (10%) | 6 (43%) | ||
| C | 0 (0%) | 4 (15%) | 0 (0%) | 0 (0%) | ||
| Stage | NS | 0.0005 | ||||
| 1 | 5 (28%) | 2 (8%) | 0 (0%) | 0 (0%) | ||
| 2 | 13 (72%) | 23 (88%) | 24 (83%) | 5 (36%) | ||
| 3 | 0 (0%) | 0 (0%) | 3 (10%) | 0 (0%) | ||
| 4 | 0 (0%) | 1 (64%) | 2 (7%) | 9 (64%) |
Surgical resection was performed in 47 patients: lobectomy (N = 28), wedge resection (N = 12), segmentectomy (N=6), and trisegmentectomy (N = 1). There were four mortalities (9%) due to: myocardial infarction (N=2), cerebrovascular accident (N=1) and hemorrhage (N=1). Local recurrence developed in one patient (2%) and new intrahepatic recurrence developed in 13 patients (28%). Nine of these 14 recurrences were subsequently treated with multi-modal therapies including transarterial chemo-embolization (N=4), systemic chemotherapy (N = 3), re-resection (N = 1), and RFA (N = 1). At the time of this analysis, 27 patients (57%) were alive. The most common reason for death was liver failure, which developed in seven patients (35%). Overall 1-, 3- and 5-year survival rates were 75%, 65%, and 31% respectively (Table III).
Table III. Local recurrence, new recurrence and overall survival based on treatment.
| Resection (N=47) | RFA (N = 40) | p-value | |
|---|---|---|---|
| Local recurrence | 1 (2%) | 4 (10%) | NS |
| New recurrence | 13 (28%) | 10 (25%) | NS |
| Overall survival | NS | ||
| 1-year | 75% | 78% | |
| 3-year | 65% | 58% | |
| 5-year | 31% | 39% | |
| Median (months) | 47 | 51 | |
RFA was performed in 40 patients by using a percutaneous (N = 24), laparoscopic (N=9) or open surgical (N=9) approach. There was one mortality (3%) secondary to liver failure. Complications were noted in seven patients (18%), including refractory ascites (N=3), pneumothorax (N=1), pneumonia (N=1), cardiac arrhythmia (N = 1) and myoglobulinemia (N = 1). Local recurrence developed in four patients (10%) and new intrahepatic recurrence developed in ten patients (25%). Seven of these 14 recurrences were subsequently treated with multimodal treatments including transarterial chemoembolization (N=5) and repeat RFA (N=2). At the time of the analysis, 25 patients (63%) were alive. All deaths were secondary to liver failure (15 out of 15, 100%). Overall 1-, 3- and 5-year survival rates were 78%, 58%, and 39% respectively (Table III).
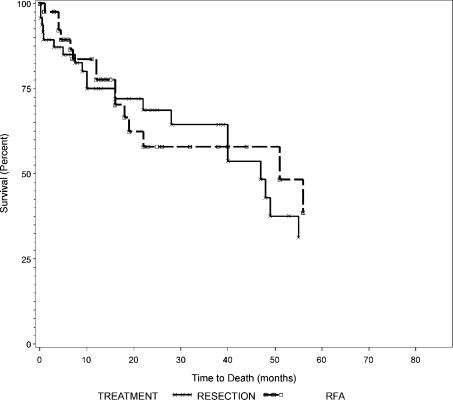
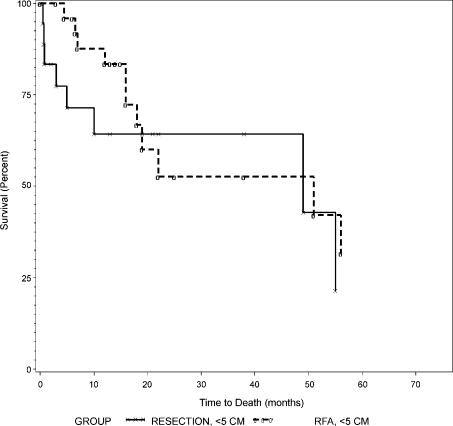
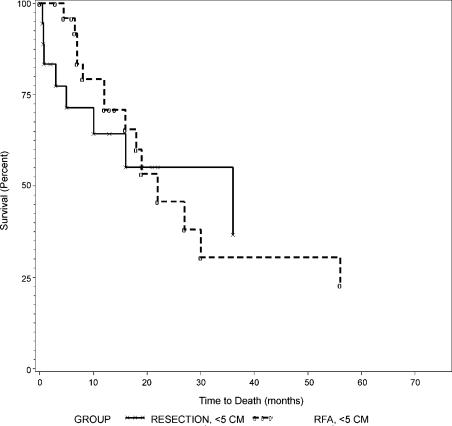
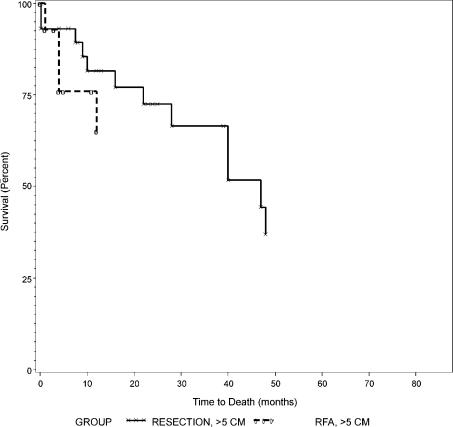
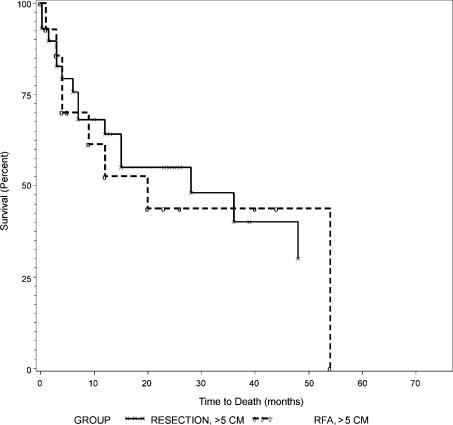
Overall survival curves between resection and RFA were not different as shown in Figure 1 (p=0.79). Overall and disease-free survival rates between those with tumors of 5 cm or less (Groups 1 and 2) and those with tumors larger than 5 cm (Groups 3 and 4) are summarized in Table IV. Both overall and disease-free survival curves were not different between Groups 1 and 2 as shown in Figures 2 and 3 (p = 0.44 and 0.84, respectively). Similarly, overall and disease-free survival curves were not different between Groups 3 and 4 as shown in Figures 4 and 5 (p=0.94 and 0.67, respectively).
Figure 1. .
Overall survival of all patients according to treatment, resection versus RFA.
Table IV. Overall and disease-free survival based on treatment and tumor size.
| Group 1 Resection ≤5 cm | Group 2 RFA ≤5 cm | p-value | Group 3 Resection >5 cm | Group 4 RFA >5 cm | p-value | |
|---|---|---|---|---|---|---|
| Number of patients | 18 | 26 | 29 | 14 | ||
| Overall survival | NS | NS | ||||
| 1-year | 64% | 83% | 82% | 65% | ||
| 3-year | 64% | 53% | 67% | 65% | ||
| 5-year | 21% | 32% | 37% | 65% | ||
| Median (months) | 49 | 51 | 47 | >63 | ||
| Disease-free survival | NS | NS | ||||
| 1-year | 64% | 71% | 64% | 53% | ||
| 3-year | 37% | 31% | 40% | 44% | ||
| 5-year | 37% | 23% | 30% | 0% | ||
| Median (months) | 36 | 22 | 28 | 20 |
Figure 2. .
Overall survival of patients with tumors ≤5 cm, Group 1 (resection) versus Group 2 (RFA).
Figure 3. .
Disease-free survival of patients with tumors ≤5 cm, Group 1 (resection) versus Group 2 (RFA).
Figure 4. .
Overall survival of patients with tumors >5 cm, Group 3 (resection) versus Group 4 (RFA).
Figure 5. .
Disease-free survival of patients with tumors >5 cm, Group 3 (resection) versus Group 4 (RFA).
Discussion
HCC is the fifth most common cancer worldwide. Although it is more prevalent in Asia and Africa, the United States has witnessed a significant increase in its incidence from 1.4 to 2.4 cases per 100,000 during the last two decades 1. Although randomized controlled studies are unavailable, surgical resection and liver transplantation are considered by most as the only potentially curative therapies 3. Long-term survival results after surgical resection have improved recently and in institutions with extensive experience, 3- and 5-year survival rates are reportedly 68%–76% and 51%–68% respectively 10. However, only selected patients are suitable for surgical resection because of advanced tumors, major vascular invasion, multifocal tumors, poor hepatic reserve or extrahepatic disease. Resection rates are low (9%–37%) even in high-volume centers 11. Liver transplant is also limited due to significant donor shortage.
In need of alternative treatments of unresectable HCC, both systemic and locoregional therapies have been investigated. Currently, among many local ablative therapies including percutaneous ethanol injection (PEI), acetic acid injection, cryotherapy, microwave coagulation, laser and radiofrequency ablation, the last has been most enthusiastically utilized. Rossi et al. 12 first described RFA of human liver tumors in a large study in the early 1990s, while the earliest recorded use of heat to treat tumors dates back to Egyptian and early Greek medical descriptions 13. RFA uses the energy of 450–500 kHz radiowaves to deliver hyper-thermic ablation to target tissue. There are a number of reasons that RFA is gaining the most popularity among all the other local ablative therapies. First, RFA has been proven to be safe by many authors with acceptable complication rates 6. With newer and larger multiple probes, larger tumors can be ablated more predictably. Also, RFA can be performed not only percutaneously but also by laparoscopic or laparotomy approach. This versatility encouraged some to consider RFA as a first-line local palliative therapy for unresectable HCC 11.
Because RFA is a relatively new treatment, the majority of the literature describes safety, local control efficacy, complication rates and early survival. There are only few studies which reported long-term survival data (Table V) 4,5,14,15,16,17. Our survival rates after RFA are comparable to previously reported long-term results, although this study included only single nodule HCC but did not exclude large (>5 cm) tumors whereas most others studied small tumors (<5 cm) including both single and multiple lesions. Most recently Vivarelli 17 reported that the overall survivals were significantly lower in RFA group than resection group; however, the RFA-treated group had significantly more patients with multiple lesions as compared to resected patients, and there was no statistical difference between the two treatments in overall survivals when patients with single nodule were compared.
Table V. Clinical studies on RFA for HCC with long-term overall survival (≥3 years).
| Author, Year | Number of patients | 1-year | 3-year | 5-year |
|---|---|---|---|---|
| Rossi, 1996 4 | 39 | 94% | 68% | 40% |
| Buscarini, 2001 5 | 88 | 89% | 62% | 33% |
| Iannitti, 2002 14 | 30 | 92% | 60% | NA |
| Guglielmi, 2003 15 | 53 | 87% | 45% | NA |
| Giovannini, 2003 16 | 56 | 96% | 96% | NA |
| Vivarelli, 2004 17 | 79 | 78% | 33% | NA |
| This study | 40 | 78% | 58% | 39% |
NA: not available.
Although these RFA results and historic controls after resection are not comparable, recent data indicate that RFA may offer equivalent survival results to surgical resection 4,5,14,15,16. In the present study, local control efficacy and long-term survival rates were not statistically different between those who underwent resection with curative intent and those who underwent RFA mostly for unresectable tumors. The overall 5-year survival of the resection group was 31% despite that neither large tumors (>5 cm) nor Child B was considered as contraindications for curative resection.
This study included only patients with single nodule HCC because tumor characteristics of patients with multiple lesions who underwent resection or RFA during the study period were rather heterogeneous. We wanted to eliminate some of the discrepancies in tumor characteristics such as bilateral versus unilateral, vascular invasion and single nodule versus multiple. Even within the group of patients with single nodule HCC, many of patients' characteristics were different. Patients undergoing RFA had a few less favorable characteristics such as age, TNM stage and Child-Pugh class likely due to the unresectable nature of HCC treated by RFA, but did have tumors that were significantly smaller. When divided into subgroups, however, the mean tumor size between Groups 1 and 2 was not different. Group 1 who underwent surgical resection for tumors of 5 cm or less, and Group 2 who underwent RFA for tumors of 5 cm or less, were more comparable. This study showed similar survival rates provided by resection versus RFA for patients with tumors of 5 cm or less (Groups 1 and 2) as well as those with tumors larger than 5 cm (Groups 3 and 4). However, the study is limited as it is retrospective and does not take into account what has been accomplished by subsequent multimodal treatments. In addition, this study evaluated a small number of patients. Because HCC is an uncommon malignancy, it is difficult to study large numbers of patients without multicenter collaboration.
Neither of these modalities, even when combined with other treatments, compares to the long-term survival following liver transplantation. Liver transplant for HCC is best performed in T1 and T2 lesions with a 5-year survival of 50%–60% 18. More recently, Tamura 19 and Jonas 20 reported 5-year survival of 78% and 71% respectively. Ultimately, the best management of HCC would involve prevention of viral hepatitis, early detection and liver transplant. Donor shortages prevent optimal management, but perhaps living donor transplant may alter this problem. Without sufficient organ donors, we are left with resection or local ablation. While this study does not define an algorithm how to use these modalities, it does point out the potential differences in patients who are candidates for each therapy. RFA allows us to treat older patients and patients with more advanced liver dysfunction, while resection allows us to treat significantly larger tumors. With the multimodality approach, similar long-term outcomes may be reached both with RFA and resection.
Is RFA equally effective as surgical resection for localized tumors of 5 cm or less? Is RFA indicated even for resectable HCC with curative intent? These are highly controversial issues and prospective randomized studies will be required to answer these questions. Curley 21 stated that although RFA does not replace standard hepatic resection, it may be combined with partial hepatectomy for patients who otherwise are not surgical candidates. Choti 22, Guglielmi 15 and Lau 23 suggested the necessity of controlled studies comparing RFA to resection. However, prospective randomized studies would be difficult to conduct, given surgeon and institutional biases and patient preferences. The present study was intended to attempt to evaluate RFA versus resection in a retrospective fashion, in the hope that multiple institutions will collaborate on retrospective data similarly in the future. Should large retrospective studies indicate similar outcomes between resection and RFA, perhaps surgeon/institution biases can be put aside for properly conducted randomized, prospective studies.
Conclusions
Although the groups were not completely comparable, this retrospective study suggests that RFA may offer similar long-term results to surgical resection for single nodule HCC when combined with multimodal treatments. Resection may be better used for a large HCC, while RFA can be performed for an unresectable HCC with advanced liver dysfunction.
References
- 1.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:74–83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma. A prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong Y, Sung RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;220:790–800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi S, Di Stasi M, Buscarini E, Cavanna L, Quaretti P, Squassante E, Garbagnati F, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am J Roentgenol. 1996;167:759–68. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 5.Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–21. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 6.Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, Furumoto NL, Oishi RH. Ultrasound-guided radio-frequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001;5:477–89. doi: 10.1016/s1091-255x(01)80085-8. [DOI] [PubMed] [Google Scholar]
- 7.Bowles BJ, Machi J, Limm WM. Safety and efficacy of radio-frequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–9. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- 8.Child CG, Turcotte JG. WB Saunders; Philadelphia: 1964. Surgery in portal hypertension. Major problems in clinical surgery: The liver and portal hypertension; p. 1. [PubMed] [Google Scholar]
- 9.Pugh RNH, Murray-Lyon IM, Dawson JL. Transection of oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–64. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 10.Broelsch CE, Frilling A, Malago M. Hepatoma resection or transplantation. Surg Clin N Am. 2004;84:495–511. doi: 10.1016/j.suc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–86. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J Interv Radiol. 1993;8:97–103. [Google Scholar]
- 13.LeVeen RF. Laser hyperthermia and radiofrequency ablation of hepatic lesions. Sem Interven Radiol. 1997;14:313–24. [Google Scholar]
- 14.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422–7. doi: 10.1001/archsurg.137.4.422. [DOI] [PubMed] [Google Scholar]
- 15.Guglielmi A, Ruzzenente A, Battocchia A, Tonon A, Fracastoro G, Cordiano C. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480–4. [PubMed] [Google Scholar]
- 16.Giovannini M, Moutardier V, Danisi C, Bories E, Pesenti C, Delpero JR. Treatment of hepatocellular carcinoma using percutaneous radiofrequency thermoablation; results and outcome in 56 patients. J Gastrointest Surg. 2003;7:791–6. doi: 10.1016/s1091-255x(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 17.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–7. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong LL. Current status of liver transplantation for hepatocellular cancer. Am J Surg. 2002;183:309–16. doi: 10.1016/s0002-9610(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 19.Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30. [PubMed] [Google Scholar]
- 20.Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma. Hepatology. 2001;33:1080–6. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 21.Curley SA. Radiofrequency ablation of malignant liver tumors. The Oncologist. 2001;6:14–23. doi: 10.1634/theoncologist.6-1-14. [DOI] [PubMed] [Google Scholar]
- 22.Choti MA. Surgical management of hepatocellular carcinoma: Resection and ablation. J Vasc Interv Radiol. 2002;13:S197–203. doi: 10.1016/s1051-0443(07)61787-4. [DOI] [PubMed] [Google Scholar]
- 23.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–9. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]