Abstract
OBJECTIVE
Leukocyte recruitment to inflammatory sites is a prominent feature of acute and chronic inflammation. Instrumental in this process is the coordinated upregulation of leukocyte integrins (among which α4β1 and β2 integrins are major players) and their cognate receptors in inflamed tissues. To avoid the ambiguity of previous short-term antibody-based studies and to allow for long-term observation, we used genetically deficient mice to compare roles of α4 and β2 integrins in leukocyte trafficking.
METHODS
Aseptic peritonitis was induced in α4 or β2 integrin-deficient (conditional and conventional knockouts, respectively) and control mice, and recruitment of major leukocyte subsets to the inflamed peritoneum was followed for up to 4 days.
RESULTS
Despite normal chemokine levels in the peritoneum and adequate numbers, optimal recruitment of myeloid cells was impaired in both α4- and β2-deficient mice. Furthermore, clearance of recruited neutrophils and macrophages was delayed in these mice. Lymphocyte migration to the peritoneum in the absence of α4 integrins was drastically decreased, both at steady state and during inflammation, a finding consistent with impaired lymphocyte in vitro adhesion and signaling. By contrast, in the absence of β2 integrins, defects in lymphocyte recruitment were only evident when peritonitis was established.
CONCLUSIONS
Our data with concurrent use of genetic models of integrin deficiency reveal non-redundant functions of α4 integrins in lymphocyte migration to the peritoneum and further refine specific roles of α4 and β2 integrins concerning trafficking and clearance of other leukocyte subsets at homeostasis and during inflammation.
INTRODUCTION
A prominent feature of acute or chronic inflammation is the recruitment of mature leukocytes to inflammatory sites. For the successful implementation of this process, several highly coordinated adhesion and activation steps need to be accomplished by leukocytes in inflammed tissues [1,2]. Essential molecular players in this multi-step adhesion/migration cascade are α4 and β2 integrins. In particular, α4β1 (VLA4) integrin is unique among integrins as it can function in all three steps of the trafficking cascade: rolling/tethering initiated by selectins, firm adhesion, and transmigration step controlled by activated integrins [3-6]. Expression of α4 integrins is constitutive in all leukocytes except human neutrophils, where it is inducible [7], whereas murine neutrophils constitutively express α4β1 [8]. The β2 integrins are expressed exclusively in hematopoietic cells [9]. Both α4 and β2 integrins, as well as their cognate receptors are up-regulated by various inflammatory stimuli [1,6].
Function-blocking antibodies and peptides have been extensively used to study the role of α4 and β2 integrins in leukocyte trafficking. However, results of antibody studies vary with the animal model used or the route of antibody administration, and off-target effects can not be excluded [10-14]. To avoid the ambiguity of antibody-based studies and to carry out long-term observations, mouse models with genetically modified integrin genes have been generated [15-17]. To circumvent embryonic lethality of α4 knockout mice [18] in studying the role of α4 integrins in vivo, reconstitution of RAG-/- mice with α4-deficient ES cells was undertaken [19]. In this model though, a profound defect in development of α4-/- lymphocytes and lymphoid organs was observed in postnatal life, thus precluding the use of this model to study migratory behavior of mature leukocyte populations. A new model of postnatal conditional α4 deficiency with normal development of the immune system was recently established in our laboratory [20].
Using this model, new aspects of the role of α4 integrins in homing and retention of hematopoietic progenitors in the bone marrow at steady state and recovery after hematopoietic stress were revealed, but trafficking patterns of mature leukocytes to the inflammatory sites have not previously been addressed or compared to other integrin-deficient mice.
To uncover unique and overlapping roles of α4 and β2 integrins in mature hematopoietic cell trafficking, we analyzed patterns of recruitment of various leukocyte subsets to the peritoneum before and after inflammation in α4 or β2 integrin-deficient mice, using a well-studied model of aseptic thioglycolate-induced peritonitis. Our results revealed intrinsic differences of migratory responses in the absence of α4 integrins in lymphoid versus myeloid subsets. Parallel studies using mice with single α4- or β2-, as well as mice with double (α4 and β2) integrin deficiency allowed fine-tuning of the roles of α4 and β2 integrins in leukocyte trafficking.
MATERIALS AND METHODS
Mice
Mice used in this study were of C57/Bl6x129 (WT, α4Δ/Δ) or C57/Bl6 (β2-/-) background, between 8 and 12 weeks of age. Wild type (WT) animals were purchased from Taconic (Germantown, NY). Beta 2 integrin-deficient mice were obtained from Dr. A. Beaudet (Baylor College, Houston, TX) [16]. MxCre+α4f/f mice were generated in our laboratory [20]. To induce α4 integrin ablation, these mice were treated neonatally with interferon inducer, poly(I:C) (three injections of 50 μl of 1mg/ml solution in phosphate-buffered saline (PBS), intraperitoneally (i.p.), 48 hours apart). MMP9-/- mice were kindly provided by Dr R. Senior (Washington University, St. Louis, MO) [21]. All animals were bred and maintained under specific pathogen-free conditions at the University of Washington. All experimental procedures were done in accordance with Institutional Animal Care and Use Committee guidelines on approved protocols.
Antibodies
Anti-α4 integrin antibody, PS/2, was purchased from Southern Biothechnology (Birmingham, AL). PE-Cy5-conjugated F4/80 (Cl:A3-1) was from AbD Serotec Ltd (Raleigh, NC). Gr-1-PE, Gr1-APC, CD45-APC, CD45-FITC, CD3-Cy-chrome, CD4-CyChrome, CD8-FITC, B220-FITC, B220-PE, B220-CyChrome, CD19-biotin, CD18-PE, streptavidin-APC, and corresponding fluorochrome-conjugated isotype-matched immunoglobulins that served as controls were purchased from BD Biosciences (San Diego, CA)
Peritoneal inflammation
Mice were injected i.p. with 1ml of 3% thioglycolate (TG) and were sacrificed by cervical dislocation 4, 16, 48 and 96 hours post injection. The peritoneal cavity was lavaged with 5 ml of ice-cold phosphate-buffered saline (PBS) containing 5mM EDTA, cells were enumerated and used for further analysis.
Chemokine measurement
Aliquots of frozen peritoneal lavage fluid were sent to Pierce Biotechnology Inc. (Woburn, MA) to measure concentration of an array of chemokines and cytokines by Searchlight Multiplex technology.
FACS analysis
Antibody-labeled cells were analyzed on a FACSCalibur (BD Biosciences, San Jose, CA) using CellQuest software.
In vitro migration
Transwell migration was performed as described elsewhere [22]. In brief, splenocytes were stained with B220-FITC and CD3-PE and 0.5 × 106 cells were transferred into the upper chambers of transwell inserts (pore size 5μm, Corning Costar, Cambridge, MA). Splenocytes were allowed to migrate through the uncoated inserts (trans-well migration), or transwells coated with bEND3 mouse endothelial cells (trans-endothelial migration) for 4 hours at 37°C towards SDF-1α(100 ng/ml, Peprotech, Rocky Hill, NJ). Cells collected from the lower chamber were enumerated by FACS. The number of events acquired from this sample was compared to that of input cells (before migration) and the percentage of migration was calculated.
Adhesion to endothelial cells
Splenocytes were isolated and labeled with CarboxyFluoroscein Succinimidyl Ester, CFSE (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, washed and brought to 2×106 cells/ml in adhesion buffer (PBS containing 1%BSA, 2mM MgCl2, 2mM CaCl2). Labeled cells were transferred into 24-well plates (1×106 cells/well) with a confluent layer of bEND3 cells either untreated or treated overnight with TNF-α (100 ng/ml, Peprotech). After a short spin (2 min., 100×g), adhesion was allowed to occur at 37°C for 40 minutes. Non-adherent cells were washed out with PBS. bEND3 cells and adherent splenocytes were trypsinized with Tryple Select (Invitrogen), washed, and the number of CFSE-positive cells was determined by FACS after 1 min. acquisition. Cell adhesion was expressed as a percent of input cells.
Actin polymerization
Actin polymerization was performed as described [23]. In brief, splenocytes (1×106 cells) were stained with CD3-FITC and B220-FITC, stimulated with 100 ng/ml SDF-1α (Peprotech) for indicated amount of time, fixed, permeabilized, and stained with phalloidin (Invitrogen). After washing with PBS/BSA, cells, gated on lymphocytes (CD3+B220+), were analyzed by FACS.
Ca2+ mobilization
Ca2+ mobilization experiments were performed as described elsewhere [24]. Splenocytes were isolated and loaded with Indo-1 (Invitrogen), washed and labeled with B220-FITC and CD3-PE antibodies. Cells were resuspended in Ca2+ buffer (PBS, 1%BSA, 1mM CaCl2, 1mM MgCl2) at 4×106 cells/ml. Change of the 395/530 fluorescence quotient was monitored over 3 min. by FACS following stimulation with SDF-1α (200 ng/ml, Peprotech) or Ionomycin (1μM, Sigma). After analysis using FloJo software, Ca2+ mobilization was expressed as a percent of the peak levels after stimulation over baseline.
Statistical analysis
Data shown are means ± standard error of mean (sem). Statistical analyses were performed using a Student t test and P values of < 0.05 were considered significant.
RESULTS
Animal models
In the present study we used genetic models of α4 (α4β1 and α4β7) or β2 (CD18) integrin deficiency. Beta 2 integrin deficient (CD18-/-) mice were generated by germ line deletion of β2 integrin gene [16]. Alpha4 integrin-deficient mice (α4Δ/Δ) were previously generated in our laboratory as conditional knockouts. These were MxCre+α4f/f mice, in which ablation of α4 integrins occurs after treatment with interferon inducer, poly(I:C) [20]. After ablation of α4 integrin in these mice, percent of α4+ cells was reduced to 3.7%±0.2% (n=11) in the bone marrow and 5.2%±0.8% (n=11) in the peripheral blood. White blood cell (WBC) counts in both α 4 integrin-deficient (19±2×103 cells/μl) and β2 integrin-deficient (49±5 × 103 cells/μl) mice were significantly higher than in control mice (8±1 × 103 cells/μl), as previously described [16,20]. In addition, we recently generated mice with both α4 and β2 integrin deficiency by interbreeding single integrin knockouts. Here we report only preliminary results with α4Δ/Δ β2-/- doubly deficient mice, since only a handful of animals were available for experiments due to difficulties in breeding.
To study unique and redundant roles of α4 and β2 integrins in leukocyte migration in vivo, we employed the aseptic peritonitis model in all mice with integrin deficiencies and monitored various leukocyte subsets by comparing their levels in circulation and in the peritoneal cavity for intervals up to 4 days.
Kinetics of migration of various leukocyte subsets in vivo
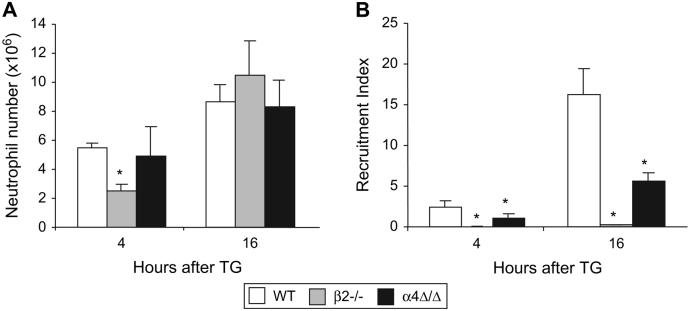
Neutrophils are the first leukocytes that migrate and accumulate at a site of inflammation. By the 4th hour of peritonitis, a substantial number of neutrophils was detected in the peritoneum of WT and α4Δ/Δ mice and increased further by the 16th hour (Fig. 1A). In β2-/- mice, neutrophils were slower to migrate, but by the 16th hour of peritonitis their numbers were comparable to those in WT animals (Fig. 1A). Defects in optimal neutrophil recruitment became evident when we compared recruitment indices in α4 and β2 integrin-deficient versus control mice (Fig. 1B). Recruitment index represents the proportion of cells harvested from the peritoneal cavity measured against the total number of cells available for migration in the circulation, assuming a blood volume of 2 ml in the mouse. The defect was more pronounced in β2-/- mice: recruitment indices at each time point were less than 2% of corresponding control value, whereas in α4Δ/Δ mice at 4 and 16 hours, they comprised 46% and 34%, respectively, of the corresponding control value. This observed defect in β2-/- neutrophil recruitment was even more striking, in view of elevated levels of neutrophil chemoattractant MIP2 in the peritoneal cavity of β2-/- mice (267±63 pg/ml) as compared to controls (74±5 pg/ml, P<0.05). In the α4-deficient mice, MIP2 levels were similar to controls, as were KC levels in both α4-/- and β2-/- mice (data not shown).
Figure 1. Both α4 and β2 integrins are required for efficient neutrophil migration to the peritoneum.
Mice were injected with TG, peritoneal leukocytes were harvested 4 and 16 hours post injection and stained with various leukocyte markers (see Materials and Methods). Neutrophil (Gr-1+,F4/80-) numbers (A) and recruitment indices (B) were calculated. Note that although sufficient numbers of neutrophils are present in the peritoneal cavity of α4Δ/Δ and β2-/- mice, there is a significant decrease in recruitment index at each time point. Asterisk (*) indicates significant difference compared to controls, P<0.05. Mice used per group: WT, n=5; β2-/-, n=4; α4Δ/Δ, n=4.
At later times of peritonitis (96 hours) more neutrophils were harvested from the peritoneum of both α4 (0.4±0.03 × 106 cells, P<0.01) and β2 (3.7±0.9 × 106 cells, P<0.01) integrin-deficient mice as compared to controls (0.1±0.02 × 106 cells).
It is worth noting that, in WT mice, all peritoneal neutrophils were α4-positive, whereas in α4- deficient mice, all migrated neutrophils were α4-negative (data not shown), suggesting their α4-independent migration.
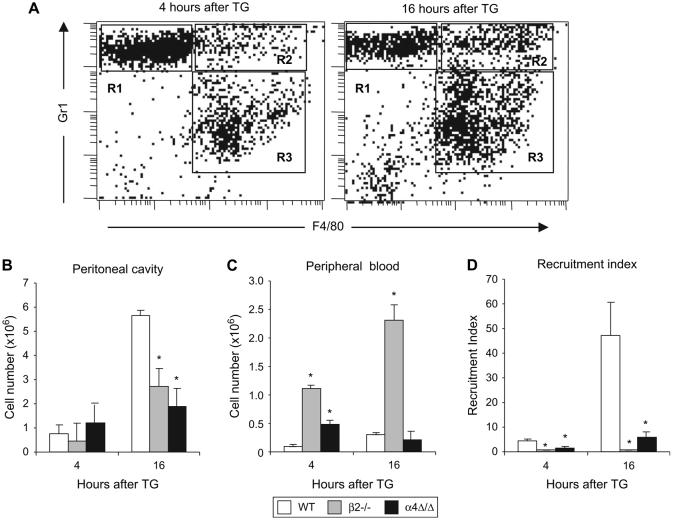
As a general rule, macrophage recruitment follows that of neutrophils during inflammation. We first analyzed inflammatory monocytes, a subset that is recognized by the presence of Gr-1 marker in addition to the monocytic marker, F4/80 [25] (Fig. 2A). Although there was no difference in the numbers of inflammatory monocytes at 4 hours, at the peak of their accumulation (16 hours), significantly fewer inflammatory monocytes were recovered from peritoneum of either α4 or β2-integrin-deficient mice than in controls (Fig. 2B), despite their increased numbers in circulation (Fig. 2C). Calculated recruitment indices further emphasized impairment in recruitment of inflammatory monocytes in integrin-deficient mice (Fig. 2D). The observed defect occurred despite normal levels of MCP1 and MIP1α, mononuclear cell chemokines, in the peritoneal cavity of these mice (data not shown).
Figure 2. Recruitment of inflammatory monocytes to the peritoneum is impaired in α4Δ/Δ and β2-/- mice.
Aseptic peritonitis was induced in mice by TG injection and 4 and 16 hours later, numbers of inflammatory monocytes were counted. (A) Typical FACS profile of peritoneal exudate of a WT mouse 4 hours (left panel) and 16 hours (right panel) after TG injection. Neutrophils are Gr-1+F4/80- (R1), inflammatory monocytes are Gr-1+F4/80+ (R2), and non-inflammatory monocytes/macrophages are Gr-1-F4/80+ (R3); Numbers of inflammatory monocytes in WT (n=5), β2-/- (n=4), and α4Δ/Δ (n=4) mice were determined in the peritoneal cavity (B) and in peripheral blood (C). Recruitment indices (D) were calculated to assess the efficiency of migration from the circulation. Asterisk (*) indicates significant difference over control (P<0.05).
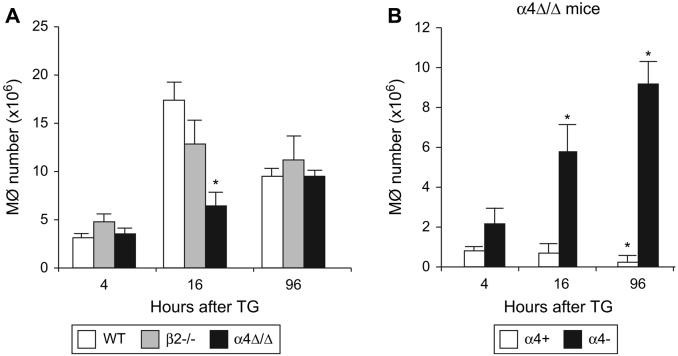
We next studied accumulation of total peritoneal macrophages (F4/80-positive cells). The numbers of F4/80+ cells recovered from the peritoneum were similar in integrin-deficient and control mice, except for a transient but significant decrease in macrophage migration at 16 hours in α4-deficient mice (Fig. 3A). Of interest, at steady state, 37.61%±3.42% of peritoneal F4/80+ macrophages were α4-positive and this proportion gradually decreased after TG injection to 32.71% ± 5.99% at 4 hours, 13.16% ± 3.98% at 16 hours, and further to 2.78% ±1.88% at 96 hours. While the decrease in proportion of α4-positive macrophages was likely attributable in part to dilution by influxing α4-negative macrophages, their total numbers also decreased with time (Fig. 3B) in α4-deficient mice.
Figure 3. Macrophages use α4 and β2 integrins interchangeably to maintain sufficient numbers in peritoneum during inflammation.
(A) Peritonitis was induced in WT (n=5), β2-/- (n=4), and α4Δ/Δ (n=4) mice and 4, 16, and 96 hours later numbers of macrophages (F4/80+) and (B) numbers of α4-positive and -negative macrophages in a4Δ/Δ peritoneal lavage were determined by FACS. Asterisk (*) indicates significant difference over control (P<0.05); Mφ denotes macrophages.
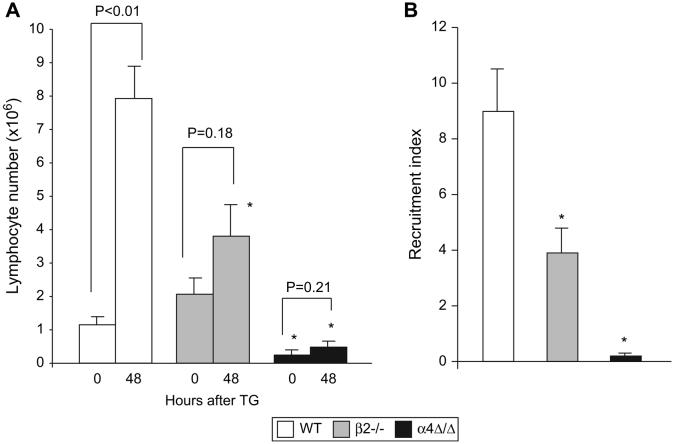
To investigate recruitment of lymphocytes, we compared numbers of T and B cells in the peritoneal lavage of naïve mice and 48 hours after TG injection, at which time the peak of lymphocyte recruitment is observed [26]. In β2-/- mice, although the lymphocyte numbers in peritoneal cavity (2.05 × 106 ±0.45 × 106 cells) were similar to controls (1.15 × 106 ± 0.19 × 106 cells) at steady state, a defect in lymphocyte recruitment became evident after inflammation was established: TG injection resulted in less than 2-fold increase over baseline in β2-/- lymphocyte numbers that is significantly less than a 7-fold increase in controls (Fig. 4A) and the recruitment index was also significantly decreased (Fig. 4B). In non-stimulated α4-deficient animals, even though only a small number was recovered from the peritoneal cavity, 47.1% ± 7.6% of T cells and 71.4% ± 8.1% of B cells were α4-positive, in contrast to 5.2% ± 0.8% of α4-positive cells present in the peripheral blood. These data suggest preferential migration and accumulation over time of α4-positive cells at steady state. Very few α4-negative lymphocytes were detected in peritoneal cavities of α4-deficient mice, and this number did not change after TG injection (Fig. 4A). The recruitment index for α4-/- lymphocytes was 45-fold less than for control (Fig. 4B), indicating a profound defect in α4-/-lymphocyte migration and recruitment. Of interest, a decrease in levels of lymphocyte chemoattractant RANTES in the peritoneal cavity was observed in α4-deficient relative to control mice (6.37 ± 1.42 pg/ml and 14.63 ± 1.65 pg/ml, respectively, P<0.05) whereas measured RANTES levels in β2-/- mice, were similar to controls (data not shown). The majority of migrated lymphocytes in all TG-induced peritonitis mouse models studied was comprised of B cells (data not shown).
Figure 4. Lymphocyte migration to the inflamed peritoneum is absent in α4Δ/Δ and decreased in β2-/- mice.
(A) Aseptic peritonitis was induced in WT (n=5), β2-/- (n=5) and α4Δ/Δ (n=5) mice. Lymphocyte numbers were determined by FACS, before and 48 hours after TG injection. (B) Efficiency of migration, as indicated by recruitment index, was calculated at 48 hours after TG injection. Asterisk (*) indicates significant difference from control mice at the corresponding hour of peritonitis (P<0.05).
In the WT mice, TG injection resulted in transient leucopenia: at 4 and 16 hours of peritonitis circulating WBC numbers dropped to 3.2±0.5 × 103 and 4.0±0.5 × 103 cells/μl blood, respectively, but returned to basal levels by the 96th hour (7.2±0.4 × 103 cells/μl blood). In contrast, in the integrin-deficient mice, no significant changes in WBC counts were detected over the course of peritonitis as compared to baseline (data not shown).
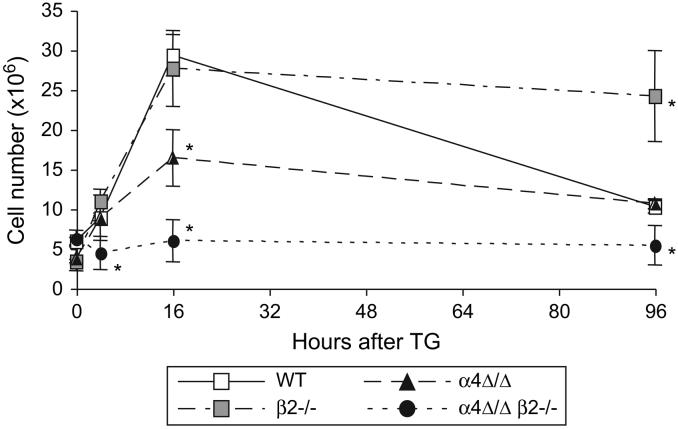
We recently developed animals with both α4 and β2 integrin deficiency by interbreeding the single integrin-deficient mice. The baseline WBC counts (109 ± 10 × 103 cells/μl, n=14) reached unprecedented levels in these doubly deficient mice, likely reflecting not only persistent inflammatory conditions, but also profound defects in leukocyte emigration to tissues. Indeed, peritoneal leukocyte numbers at 0, 4, 16 and 96 hours after TG injection were 5.6 ± 0.3 × 106 (n=4), 4.4 ± 2.1 × 106 (n=4), 5.9 ± 2.6 × 106 (n=3), and 5.4 ± 2.4 × 106 (n=3) cells respectively, indicating abrogation of leukocyte recruitment in the absence of both integrins, despite markedly elevated circulating WBCs (Fig. 5). Comparison of leukocyte recruitment in single integrin- and double-deficient animals indicates that both integrins are absolutely required for leukocyte migration to the peritoneal cavity.
Figure 5. Alpha4 and beta2 integrins are necessary and sufficient for leukocyte migration to the inflamed peritoneum.
Total leukocyte numbers in peritoneum of WT (n=5), β2-/- (n=5), α4Δ/Δ (n=5), and α4Δ/Δ β2-/- double deficient (n=4) mice at various times after induction of aseptic peritonitis. Note that, most of the time leukocyte numbers in mice deficient in a single integrin are similar to that of controls, whereas no recruitment of double deficient leukocytes occurs. Asterisk (*) indicates significant difference from control (P<0.05)
In vitro functional testing of α4Δ/Δ lymphocytes
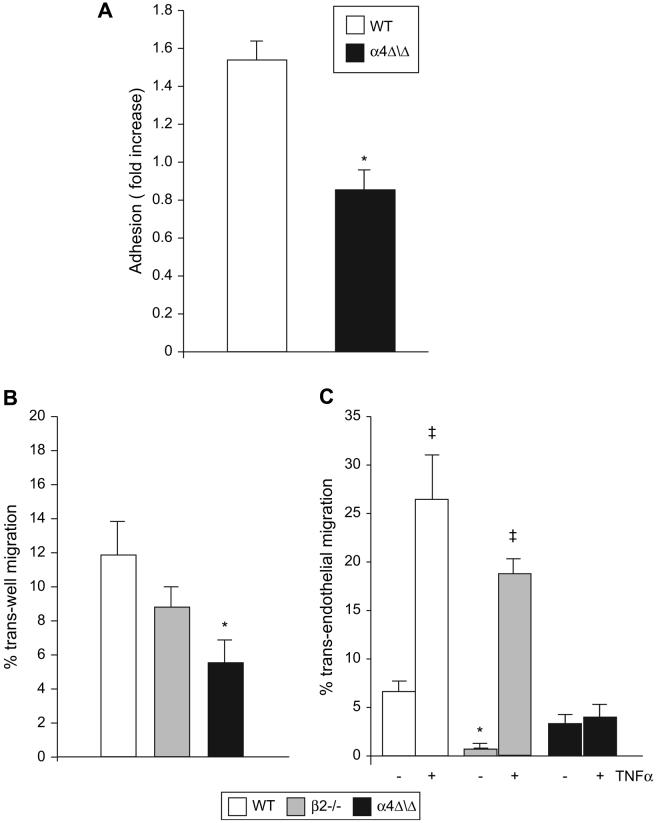
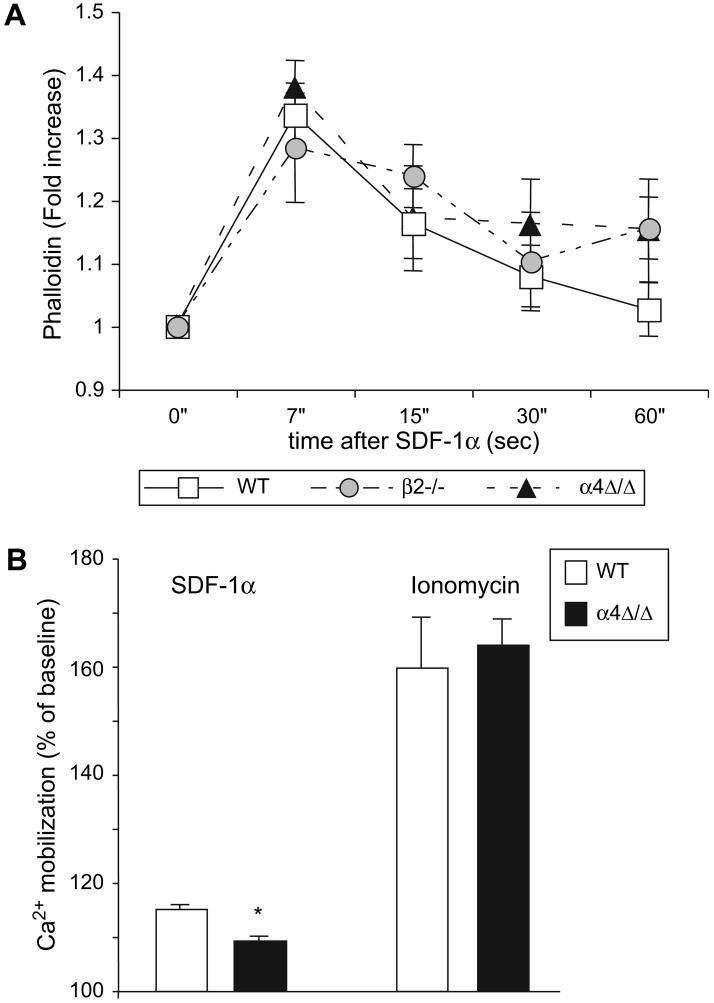
Considering that the most prominent defect in recruitment to the inflamed peritoneum involved the α4Δ/Δ lymphocytes, we further investigated their migratory behavior in vitro. We compared adhesion of control and α4Δ/Δ lymphocytes to TNFα-stimulated (VCAM-1 expressing) and non-stimulated bEND3 cells, a mouse endothelial cell line. Twice as many control lymphocytes adhered to VCAM-1-expressing bEND3 cells as compared to non-stimulated cells; at the same time, no increase in adhesion was observed with α4Δ/Δ lymphocytes (Fig. 6A). These data suggest the preferential usage of α4 integrins by lymphocytes for adhesion to bEND3 cells, since TNFα not only up-regulates VCAM-1 but also ICAM-1, a β2 integrin ligand on endothelial cells [27,28]. To determine whether ability to migrate per se is preserved in α4Δ/Δ lymphocytes, we performed migration through an uncoated Transwell insert (trans-well migration) not requiring engagement of α4 integrins. As a stimulus for migration we used SDF-1α, a well-characterized lymphocyte chemoattractive agent [29]. In this setting, since equal amounts of chemoattractant are present, differences in migration would reflect cell intrinsic defects. Substantial migration of B- and T-lymphocytes in all genotypes except for α4Δ/Δ B-cells was observed (Fig. 6B and data not shown), suggesting impairment of signaling in these cells. We next performed lymphocyte migration through a Transwell coated with bEND3 cells (trans-endothelial migration). Lymphocytes were allowed to migrate through non-stimulated bEND3 cells or cells stimulated with TNFα upregulating VCAM-1. Control and β2-/- B-cells migrated more efficiently through TNFα-treated endothelium; in contrast, α4Δ/Δ B-cells showed no increase in migration through VCAM1-expressing bEND3 cells (Fig. 6C). Similar results were seen with T-cells (data not shown). Of interest, significantly fewer β2-/- B-cells migrated through the non-stimulated endothelial layer, whereas their migration through VCAM-1-expressing bEND3 cells was similar to control indicating that migration through the TNFα-stimulated endothelial cells is superseded by α4 integrins via VLA4/VCAM-1 interaction. Since trans-well migration was impaired in α4Δ/Δ lymphocytes, we tested early signaling events characteristic of this process. Actin polymerization is a very early event in migration-associated signaling in response to SDF-1α. No significant differences in actin polymerization, measured by phalloidin staining, were detected in either integrin-deficient lymphocytes or control cells, suggesting preservation of proximal signaling steps leading to actin remodeling (Fig. 7A). At the same time, absence of α4 integrin in lymphocytes resulted in a modest but significant decrease in SDF-1α-induced Ca2+ mobilization (Fig. 7B), likely reflecting impaired signaling. These data suggest impaired response of α4-deficient lymphocytes to soluble chemoattractants.
Figure 6. Alpha-4 integrins are essential for lymphocyte adhesion and migration in vitro.
(A) Adhesion of control (n=5) and α4Δ/Δ (n=3) splenocytes to endothelial cells. Splenocytes were labeled with CFSE and allowed to adhere to either non-stimulated or TNFα-stimulated bEND3 monolayer. Fold increase of adhesion to stimulated over non-stimulated endothelium is displayed. (B) Trans-well and (C) trans-endothelial migration of B-lymphocytes towards SDF-1α. Note that migration of α4Δ/Δ B cells (and T cells, not shown) through VCAM-1-expressing endothelium (TNFα) did not differ from that of non-stimulated endothelium, in contrast to control and β2-/- cells. Asterisk (*) indicates significant difference over controls; ‡ indicates significant difference over non-stimulated endothelium (P<0.05). Three mice per group were used.
Figure 7. Lymphocyte early responses to chemoattractant SDF-1α.
(A) Actin polymerization in WT, α4Δ/Δ and β2-/- lymphocytes. Splenocytes were stained for lymphocyte markers (CD3 and B220), stimulated with SDF-1α for indicated amount of time and phalloidin staining was performed. Fold increase over non-stimulated cells was calculated. (B) SDF-1α-induced Ca2+ mobilization. Splenocytes were loaded with Indo-1 and stained for lymphocyte markers. Ca2+ flux was assessed following stimulation with SDF-1α or ionomycin. Results are displayed as percent of the peak stimulation levels over baseline. Asterisk (*) indicates significant difference over control (P<0.05). Three mice per group were used.
DISCUSSION
Although the lack of either α4 or β2 integrin resulted in decreased efficiency of leukocyte recruitment to the inflamed peritoneum, the substantial numbers of total leukocytes observed at various times after TG injection emphasize the overlapping roles of α4 and β2 integrins in total leukocyte migration. These data are consistent with previous studies showing that only simultaneous functional blockade of α4 and β2 integrins (either by treatment of wild type mice with a combination of anti-α4 and anti-β2 antibodies, or by treatment of β2-deficient mice with anti-α4-antibody) prevents leukocyte recruitment to the peritoneum [15,30,31]. Our data with α4/β2 doubly deficient mice support and extend these observations. In addition, they provide direct evidence for an absence of significant compensation by other cytoadhesins [32,33] in these animals.
Our studies of the different leukocyte subsets in genetically deficient animals further delineate distinct versus overlapping contributions of α4 and β2 integrins to leukocyte subset-specific migration.
Although the presence of either α4 or β2 integrins afforded significant migration of neutrophils to inflamed peritoneum (Fig. 1A), assessment of recruitment indices (Fig. 1B) indicates that optimal recruitment requires the presence of both α4 and β2 integrins. The interchangeable usage of α4 or β2 integrins by neutrophils may be tissue-specific. While neutrophil migration to the heart and lung tissues is mediated by both α4 and β2 integrins [34,35], migration of neutrophils to the skin in non-allergic inflammation depends primarily on β2 integrins [17,36]. Tissue-specific variations in chemokine secretion and expression of adhesion molecules in local inflammatory milieu may explain different patterns of integrin usage by leukocytes during their migration to the various tissues [37].
Of interest are observations regarding the recruitment of monocyte/macrophages in α4-deficient mice. Macrophage numbers in these mice, although low in early development of peritonitis (Fig. 3A) due to delayed recruitment of inflammatory monocytes to the site of inflammation (Fig. 2), continually increased up to 96 hours (Fig. 3B). As the number of α4-negative macrophages increased, the number of α4-positive macrophages progressively decreased during the course of inflammation (Fig. 3B). The decline in total numbers did not simply reflect “dilution” by the incoming α4-negative cells, but is best explained by their egress from the peritoneal cavity, as was previously shown for normal macrophages [38]. Macrophage egress from the peritoneum observed in control mice (compare the drop in macrophage numbers between 16 and 96 hours of peritonitis, Fig. 3A) was impaired in both α4 and β2 integrin-deficient mice, suggesting that the presence of α4 integrins, along with the β2-integrin Mac1 [39], is important in their egress from inflammatory sites.
In contrast to macrophages which migrate from the peritoneal cavity into the draining lymphatics [38], neutrophils undergo apoptosis and are cleared from the peritoneum by macrophages. Thus, increased neutrophil numbers observed in both α4Δ/Δ and β2-/- mice at 96 hours post TG injection may suggest either delay in apoptosis, impaired clearance, or sustained neutrophil recruitment. Involvement of both α4 and β2 integrins in regulating apoptosis and/or clearing of apoptotic cells have been demonstrated; deficiency in β2 integrins leads to a delay in neutrophil apoptosis and their subsequent accumulation in the peritoneum [40,41], while α4 integrin on apoptotic cells assist in their phagocytosis [42]. Thus, absence of α4 integrins may affect clearance of α4Δ/Δ neutrophils, thereby resulting in increased neutrophil presence in the peritoneum.
Lymphocyte migration to the inflamed peritoneum was greatly impaired only in α4 integrin-deficient mice (Fig. 4). Our results are in agreement with previously reported observations in chimeric mice in which α4Δ/Δ lymphocytes did not migrate into the inflamed peritoneum [19]. However, the dependence of lymphocyte recruitment on α4 integrin is also a tissue-specific phenomenon; recruitment of lymphocytes to brain tissue is mainly dependent on α4 integrin [43], whereas their migration to inflamed lungs and airways is mediated by both α4 and β2 integrins [11,44,45], and migration to inflamed skin depends exclusively upon β2 integrin engagement [46].
It is important to emphasize that, beyond the complete and partial impairment in lymphocyte recruitment seen in α4 and β2-deficient mice, respectively, the defects were manifested differently: α4Δ/Δ lymphocytes showed defective migration to the non-inflamed as well as to the inflamed peritoneum; the defect in β2-/- lymphocytes was partial and became apparent only after inflammation was established (Fig. 4A). This finding is in agreement with preferential usage of α4 over β2 integrin by lymphocytes for adhesion to the extracellular matrix before and after inflammatory stumulus [47]. However, whether integrin-specific recruitment changes under shear stress is unclear. For example, preferential usage of β2 integin by T-lymphocytes in response to chemoattractive stimuli under shear stress conditions has been reported [48]. In addition, considering pre-existing high systemic levels of inflammatory cytokines in CD18-/- mice [49], the partial defect seen in β2-/- lymphocytes may also be attributed to an attenuated response to TG of already activated CD18-/- lymphocytes by pre-existing inflammatory stimuli.
The reasons for impaired α4Δ/Δ lymphocyte recruitment to the inflamed peritoneum are attributable mainly to intrinsic cell defects, rather than being dictated by the environment. This is supported by the fact that α4Δ/Δ lymphocytes showed impaired adhesion and migration to SDF-1α (both trans-well and trans-endothelial migration) (Fig. 6B, C). Consistent with these results, perturbed α4 integrin/paxillin-dependent signaling also leads to defective lymphocyte migration to the inflamed peritoneum [26]. Beyond the intrinsic migratory defects of a4Δ/Δlymphocytes, suboptimal stimulation by environmental factors (i.e. decreased levels of RANTES) cannot be excluded. The metalloproteinases (MMP) that are thought to contribute to leukocyte recruitment [50,51] most likely do not play a major role in the model employed, as we detected neither lack of induction of either MMP9 or MMP2 in the peritoneal cavity during peritonitis, nor any difference in trans-endothelial migration of MMP9-/- lymphocytes compared to controls (data not shown). This is in agreement with the observation of Betsuyaku et. al., that MMP9 is not required for efficient leukocyte migration [52].
In summary, our results demonstrate that, although critically dependent on the expression of both α4 and β2 integrins, there is a subset-specific usage of these adhesion molecules by leukocytes in their migration to and egress from the inflamed peritoneum. While the roles of α4 and β2 integrins are partially redundant in myeloid cells, they are distinct and non-overlapping in lymphocytes. Taken together, our data with genetically deficient mouse models extend and refine previous knowledge on the kinetics of leukocyte accumulation and their egress from the peritoneum in the aseptic peritonitis model. Whether kinetic changes in inflammatory models of other tissues are different will require further studies.
ACKNOWLEDGMENTS
This work was supported by the NIH grants (HL58734, DK46557) for T.P. Authors thank Ms. Devra Batdorf for expert mouse handling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- 2.Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thromb Haemost. 2003;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- 3.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193:1149–1158. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- 5.Johnston B, Issekutz TB, Kubes P. The alpha 4-integrin supports leukocyte rolling and adhesion in chronically inflamed postcapillary venules in vivo. J Exp Med. 1996;183:1995–2006. doi: 10.1084/jem.183.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–167. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- 7.Ibbotson GC, Doig C, Kaur J, et al. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat Med. 2001;7:465–470. doi: 10.1038/86539. [DOI] [PubMed] [Google Scholar]
- 8.Pereira S, Zhou M, Mocsai A, Lowell C. Resting murine neutrophils express functional alpha 4 integrins that signal through Src family kinases. J Immunol. 2001;166:4115–4123. doi: 10.4049/jimmunol.166.6.4115. [DOI] [PubMed] [Google Scholar]
- 9.Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. doi: 10.1016/s0002-9343(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 10.Mulligan MS, Lentsch AB, Miyasaka M, Ward PA. Cytokine and adhesion molecule requirements for neutrophil recruitment during glycogen-induced peritonitis. Inflamm Res. 1998;47:251–255. doi: 10.1007/s000110050326. [DOI] [PubMed] [Google Scholar]
- 11.Gascoigne MH, Holland K, Page CP, et al. The effect of anti-integrin monoclonal antibodies on antigen-induced pulmonary inflammation in allergic rabbits. Pulm Pharmacol Ther. 2003;16:279–285. doi: 10.1016/S1094-5539(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 12.Henderson WR, Jr., Chi EY, Albert RK, et al. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–3092. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham WM, Sielczak MW, Ahmed A, et al. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest. 1994;93:776–787. doi: 10.1172/JCI117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham WM, Gill A, Ahmed A, et al. A small-molecule, tight-binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am J Respir Crit Care Med. 2000;162:603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- 15.Henderson RB, Lim LH, Tessier PA, et al. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med. 2001;194:219–226. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scharffetter-Kochanek K, Lu H, Norman K, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizgerd JP, Kubo H, Kutkoski GJ, et al. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med. 1997;186:1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 19.Arroyo AG, Taverna D, Whittaker CA, et al. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for alpha 5, alpha v, and alpha 4 integrins. J Immunol. 2000;165:4667–4675. doi: 10.4049/jimmunol.165.8.4667. [DOI] [PubMed] [Google Scholar]
- 20.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol. 1998;28:1959–1969. doi: 10.1002/(SICI)1521-4141(199806)28:06<1959::AID-IMMU1959>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Papayannopoulou T, Priestley GV, Bonig H, Nakamoto B. The role of G-protein signaling in hematopoietic stem/progenitor cell mobilization. Blood. 2003;101:4739–4747. doi: 10.1182/blood-2002-09-2741. [DOI] [PubMed] [Google Scholar]
- 24.Bonig H, Rohmer L, Papayannopoulou T. Long-term functional impairment of hemopoietic progenitor cells engineered to express the S1 catalytic subunit of pertussis toxin. Exp Hematol. 2005;33:689–698. doi: 10.1016/j.exphem.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 26.Feral CC, Rose DM, Han J, et al. Blocking the alpha 4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J Clin Invest. 2006;116:715–723. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuldo JM, Westra J, Asgeirsdottir SA, et al. Differential effects of NF-{kappa}B and p38 MAPK inhibitors and combinations thereof on TNF-{alpha}- and IL-1{beta}-induced proinflammatory status of endothelial cells in vitro. Am J Physiol Cell Physiol. 2005;289:C1229–C1239. doi: 10.1152/ajpcell.00620.2004. [DOI] [PubMed] [Google Scholar]
- 28.Min JK, Kim YM, Kim SW, et al. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol. 2005;175:531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 29.Ding Z, Xiong K, Issekutz TB. Chemokines stimulate human T lymphocyte transendothelial migration to utilize VLA-4 in addition to LFA-1. J Leukoc Biol. 2001;69:458–466. [PubMed] [Google Scholar]
- 30.Winn RK, Harlan JM. CD18-independent neutrophil and mononuclear leukocyte emigration into the peritoneum of rabbits. J Clin Invest. 1993;92:1168–1173. doi: 10.1172/JCI116686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 32.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol. 1994;125:471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowden RA, Ding ZM, Donnachie EM, et al. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- 35.Burns JA, Issekutz TB, Yagita H, Issekutz AC. The alpha 4 beta 1 (very late antigen (VLA)-4, CD49d/CD29) and alpha 5 beta 1 (VLA-5, CD49e/CD29) integrins mediate beta 2 (CD11/CD18) integrin-independent neutrophil recruitment to endotoxin-induced lung inflammation. J Immunol. 2001;166:4644–4649. doi: 10.4049/jimmunol.166.7.4644. [DOI] [PubMed] [Google Scholar]
- 36.Mizgerd JP, Bullard DC, Hicks MJ, Beaudet AL, Doerschuk CM. Chronic inflammatory disease alters adhesion molecule requirements for acute neutrophil emigration in mouse skin. J Immunol. 1999;162:5444–5448. [PubMed] [Google Scholar]
- 37.Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003;134:431–441. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellingan GJ, Xu P, Cooksley H, et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinmann P, Scharffetter-Kochanek K, Forlow SB, Peters T, Walzog B. A role for apoptosis in the control of neutrophil homeostasis in the circulation: insights from CD18-deficient mice. Blood. 2003;101:739–746. doi: 10.1182/blood-2002-01-0239. [DOI] [PubMed] [Google Scholar]
- 41.Coxon A, Rieu P, Barkalow FJ, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JD, Hess KL, CookMills JM. CD44, alpha(4) integrin, and fucoidin receptor-mediated phagocytosis of apoptotic leukocytes. J Leukoc Biol. 2003;74:810–820. doi: 10.1189/jlb.0303092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deloire MS, Touil T, Brochet B, Dousset V, Caille JM, Petry KG. Macrophage brain infiltration in experimental autoimmune encephalomyelitis is not completely compromised by suppressed T-cell invasion: in vivo magnetic resonance imaging illustration in effective anti-VLA-4 antibody treatment. Mult Scler. 2004;10:540–548. doi: 10.1191/1352458504ms1090oa. [DOI] [PubMed] [Google Scholar]
- 44.Xu B, Wagner N, Pham LN, et al. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–1267. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banerjee ER, Jiang Y, Henderson WR, Scott LM, Papayannopoulou T. a4 and b2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only a4 is critical. Exp Hematol. 2007;35:605–617. doi: 10.1016/j.exphem.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 46.Grabbe S, Varga G, Beissert S, et al. Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells. J Clin Invest. 2002;109:183–192. doi: 10.1172/JCI11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solpov A, Shenkman B, Vitkovsky Y, et al. Platelets enhance CD4+ lymphocyte adhesion to extracellular matrix under flow conditions: role of platelet aggregation, integrins, and non-integrin receptors. Thromb Haemost. 2006;95:815–821. [PubMed] [Google Scholar]
- 48.Schreiber TH, Shinder V, Cain DW, Alon R, Sackstein R. Shear flow-dependent integration of apical and subendothelial chemokines in T-cell transmigration: implications for locomotion and the multistep paradigm. Blood. 2007;109:1381–1386. doi: 10.1182/blood-2006-07-032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Prince JE, Brayton CF, et al. Host resistance of CD18 knockout mice against systemic infection with Listeria monocytogenes. Infect Immun. 2003;71:5986–5993. doi: 10.1128/IAI.71.10.5986-5993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolaczkowska E, Chadzinska M, Scislowska-Czarnecka A, Plytycz B, Opdenakker G, Arnold B. Gelatinase B/matrix metalloproteinase-9 contributes to cellular infiltration in a murine model of zymosan peritonitis. Immunobiology. 2006;211:137–148. doi: 10.1016/j.imbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Renckens R, Roelofs JJ, Florquin S, et al. Matrix metalloproteinase-9 deficiency impairs host defense against abdominal sepsis. J Immunol. 2006;176:3735–3741. doi: 10.4049/jimmunol.176.6.3735. [DOI] [PubMed] [Google Scholar]
- 52.Betsuyaku T, Shipley JM, Liu Z, Senior RM. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am J Respir Cell Mol Biol. 1999;20:1303–1309. doi: 10.1165/ajrcmb.20.6.3558. [DOI] [PubMed] [Google Scholar]