Abstract
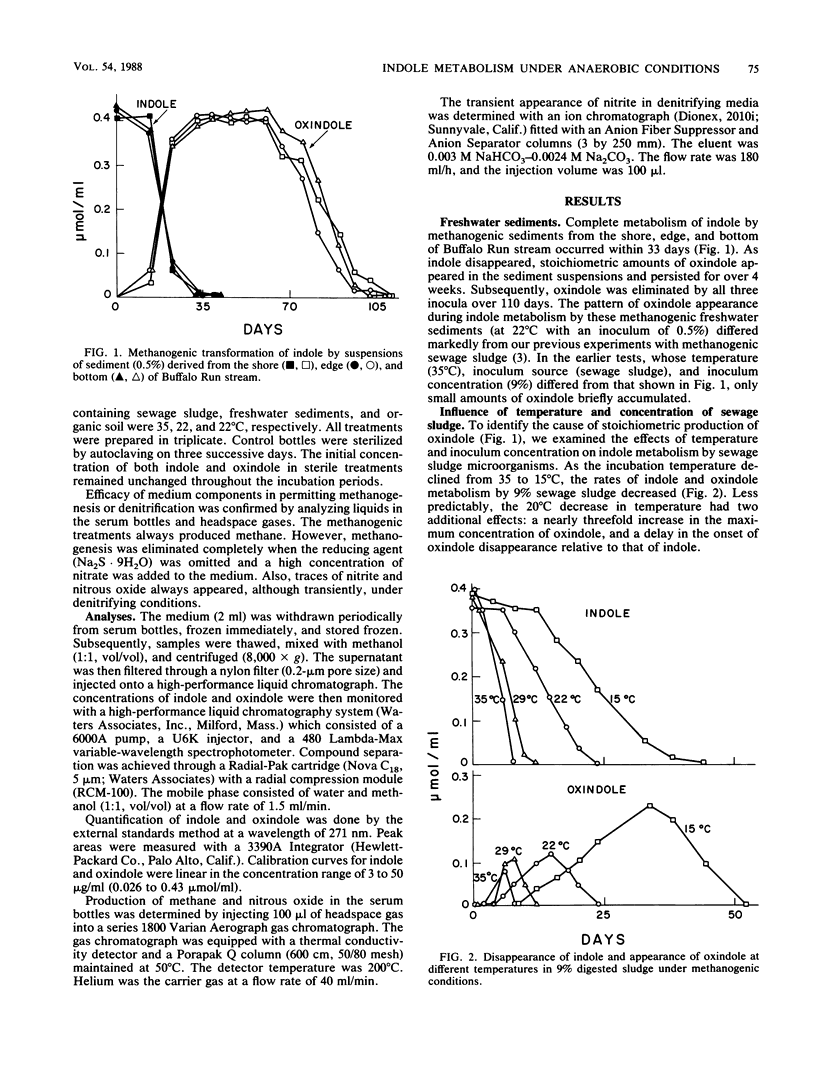
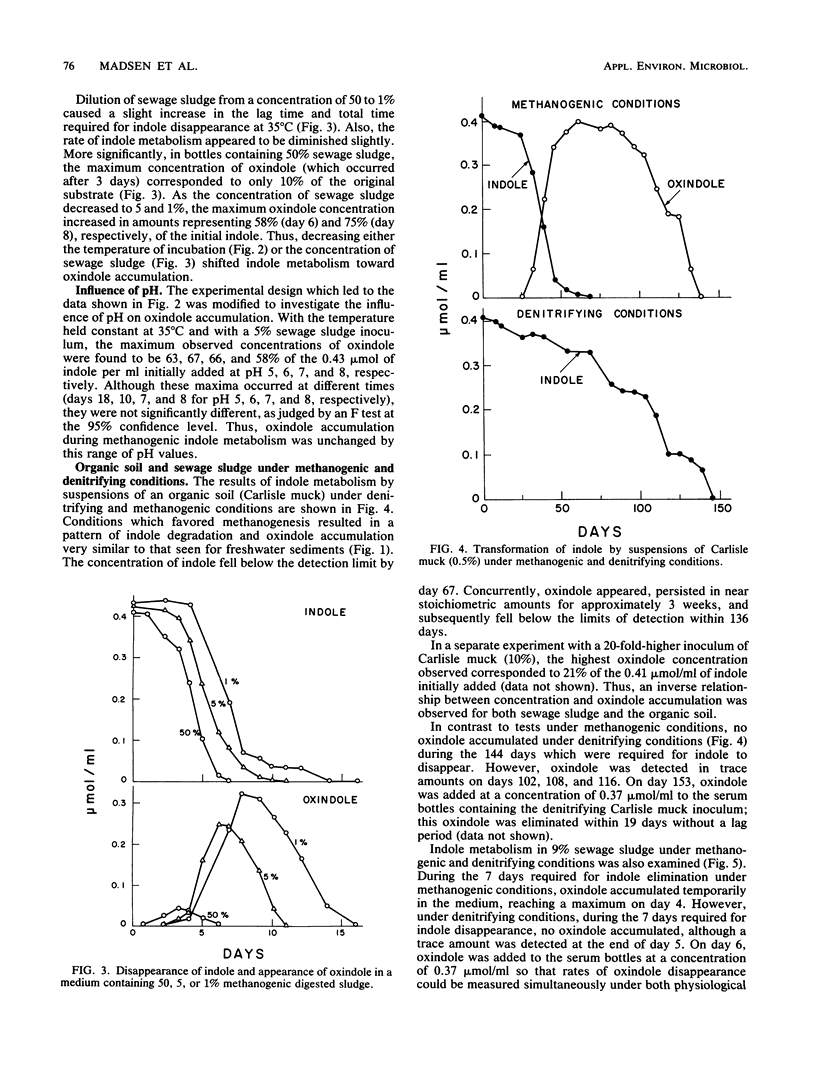
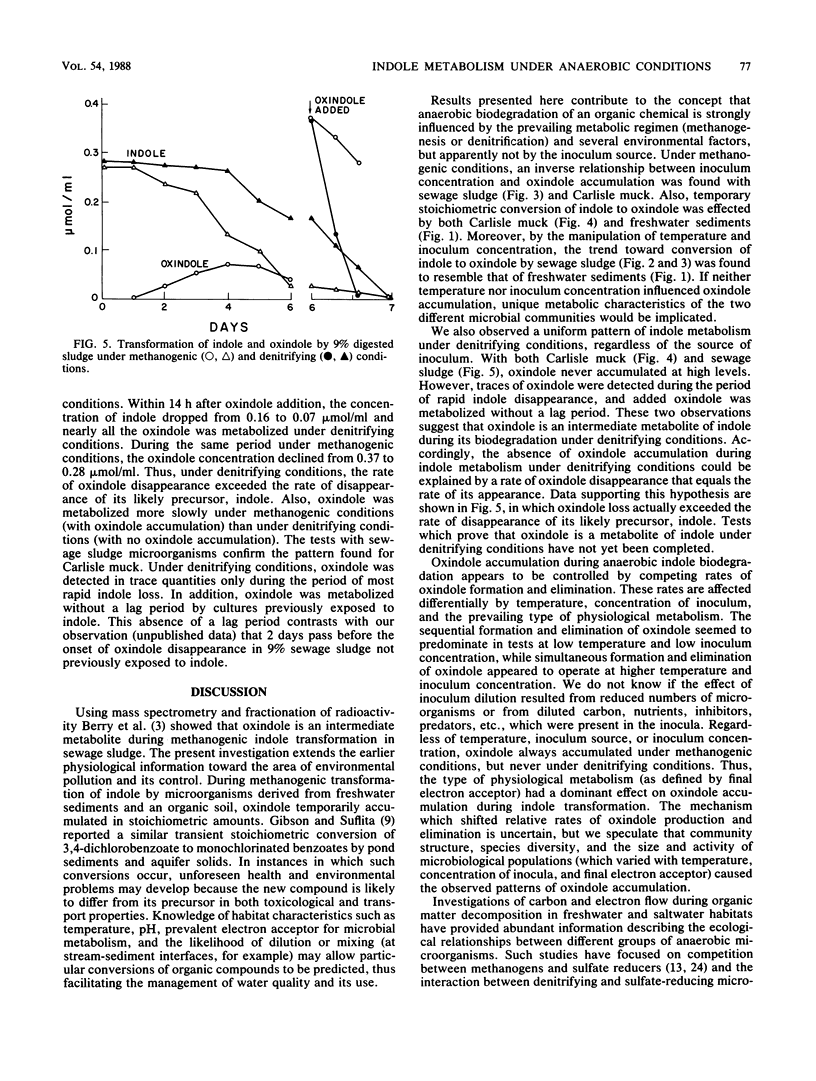
The influence of physiological and environmental factors on the accumulation of oxindole during anaerobic indole metabolism was investigated by high-performance liquid chromatography. Under methanogenic conditions, indole was temporarily converted to oxindole in stoichiometric amounts in media inoculated with three freshwater sediments and an organic soil. In media inoculated with methanogenic sewage sludge, the modest amounts of oxindole detected at 35 degrees C reached higher concentrations and persisted longer when the incubation temperature was decreased from 35 to 15 degrees C. Also, decreasing the concentration of sewage sludge used as an inoculum from 50 to 1% caused an increase in the accumulation of oxindole from 10 to 75% of the indole added. Under denitrifying conditions, regardless of the concentration or source of the inoculum, oxindole appeared in trace amounts but did not accumulate during indole metabolism. In addition, denitrifying consortia which previously metabolized indole degraded oxindole with no lag period. Our data suggest that oxindole accumulation under methanogenic, but not under denitrifying conditions is caused by differences between relative rates of oxindole production and destruction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. Methanogenic fermentation of the naturally occurring aromatic amino acids by a microbial consortium. Biochem Soc Trans. 1980 Oct;8(5):625–627. doi: 10.1042/bst0080625. [DOI] [PubMed] [Google Scholar]
- Berry D. F., Francis A. J., Bollag J. M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol Rev. 1987 Mar;51(1):43–59. doi: 10.1128/mr.51.1.43-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. F., Madsen E. L., Bollag J. M. Conversion of indole to oxindole under methanogenic conditions. Appl Environ Microbiol. 1987 Jan;53(1):180–182. doi: 10.1128/aem.53.1.180-182.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of halogenated organic compounds under denitrification conditions. Appl Environ Microbiol. 1983 Apr;45(4):1295–1299. doi: 10.1128/aem.45.4.1295-1299.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K., Gibson D. T. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol. 1984 Jul;48(1):102–107. doi: 10.1128/aem.48.1.102-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr Reductive degradation of pyrimidines. I. The isolation and characterization of a uracil fermenting bacterium, Clostridium uracilicum nov. spec. J Bacteriol. 1957 Feb;73(2):220–224. doi: 10.1128/jb.73.2.220-224.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić-Galić D., Young L. Y. Methane fermentation of ferulate and benzoate: anaerobic degradation pathways. Appl Environ Microbiol. 1985 Aug;50(2):292–297. doi: 10.1128/aem.50.2.292-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa Z., Grusenmeyer S., Verstraete W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: microbiological aspects. Appl Environ Microbiol. 1986 Mar;51(3):580–587. doi: 10.1128/aem.51.3.580-587.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenneman G. E., McInerney M. J., Knapp R. M. Effect of nitrate on biogenic sulfide production. Appl Environ Microbiol. 1986 Jun;51(6):1205–1211. doi: 10.1128/aem.51.6.1205-1211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTAN I., TSAI L., STADTMAN E. R. NICOTINIC ACID METABOLISM. I. DISTRIBUTION OF ISOTOPE IN FERMENTATION PRODUCTS OF LABELLED NICOTINIC ACID. J Biol Chem. 1964 Mar;239:902–906. [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Stadtman T. C., Pastan I., Smith L. D. Clostridium barkeri sp. n. J Bacteriol. 1972 May;110(2):758–760. doi: 10.1128/jb.110.2.758-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. T., Suidan M. T., Pfeffer J. T. Anaerobic biodegradation of indole to methane. Appl Environ Microbiol. 1984 Nov;48(5):1058–1060. doi: 10.1128/aem.48.5.1058-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



