Abstract
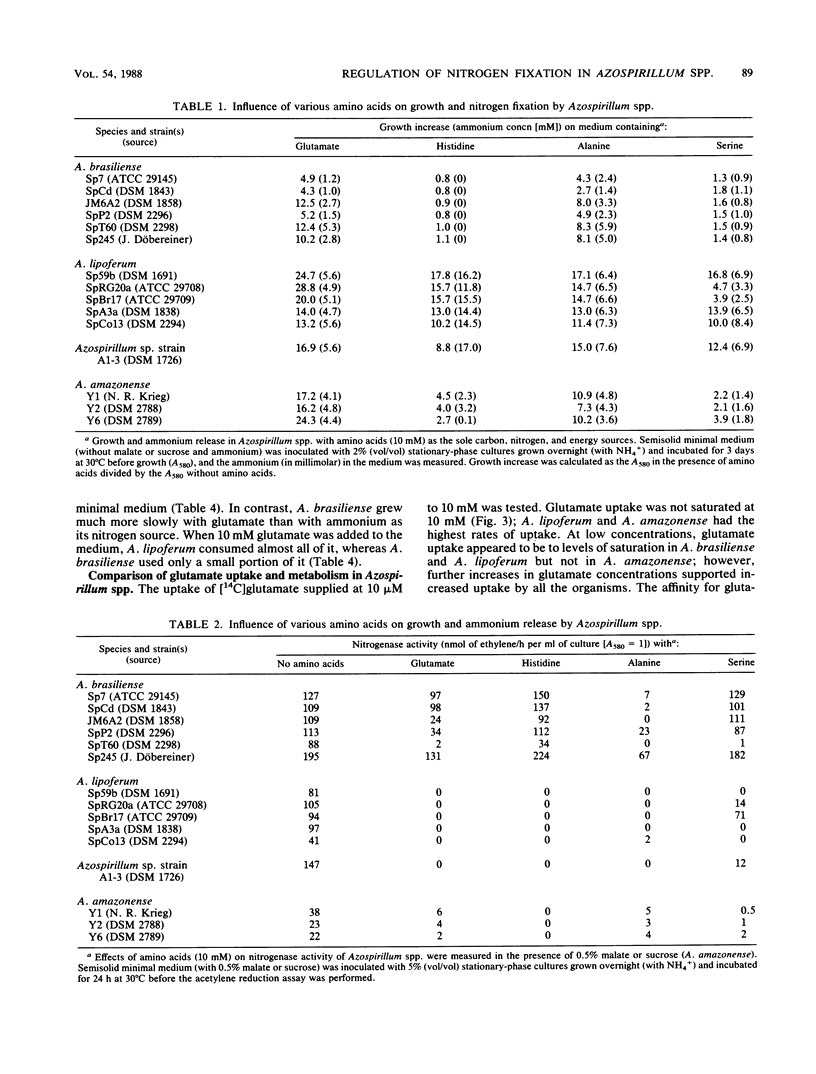
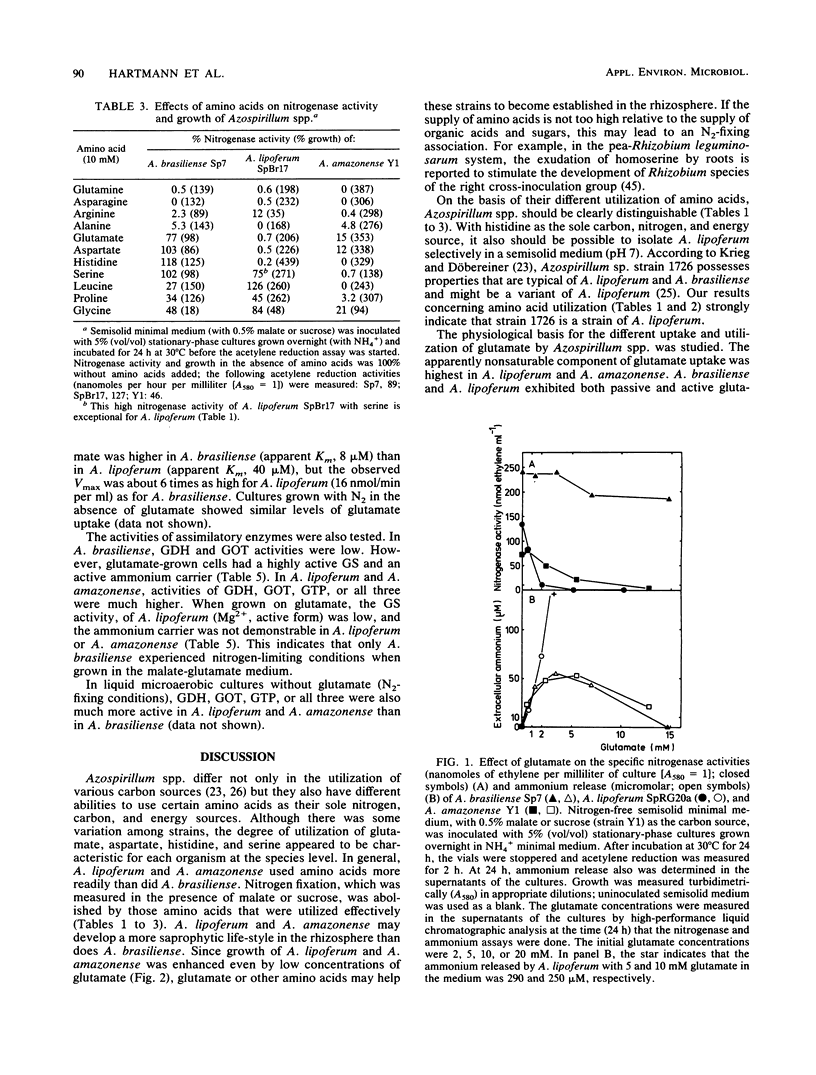
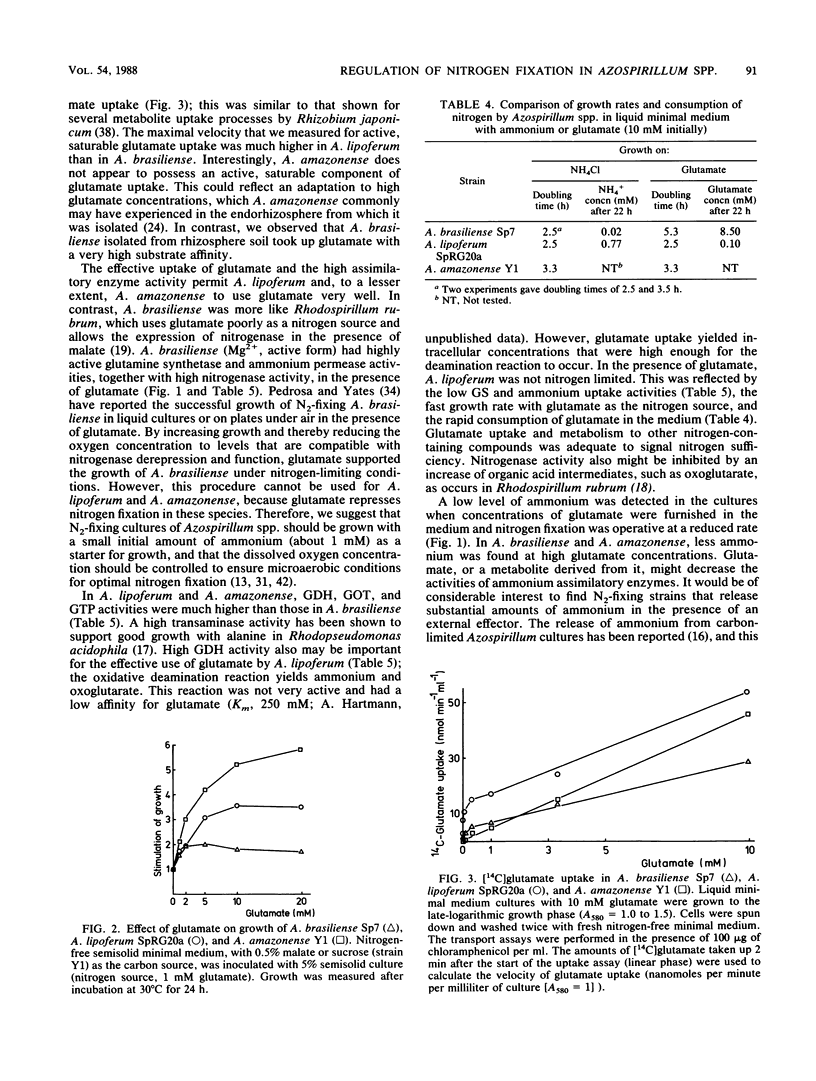
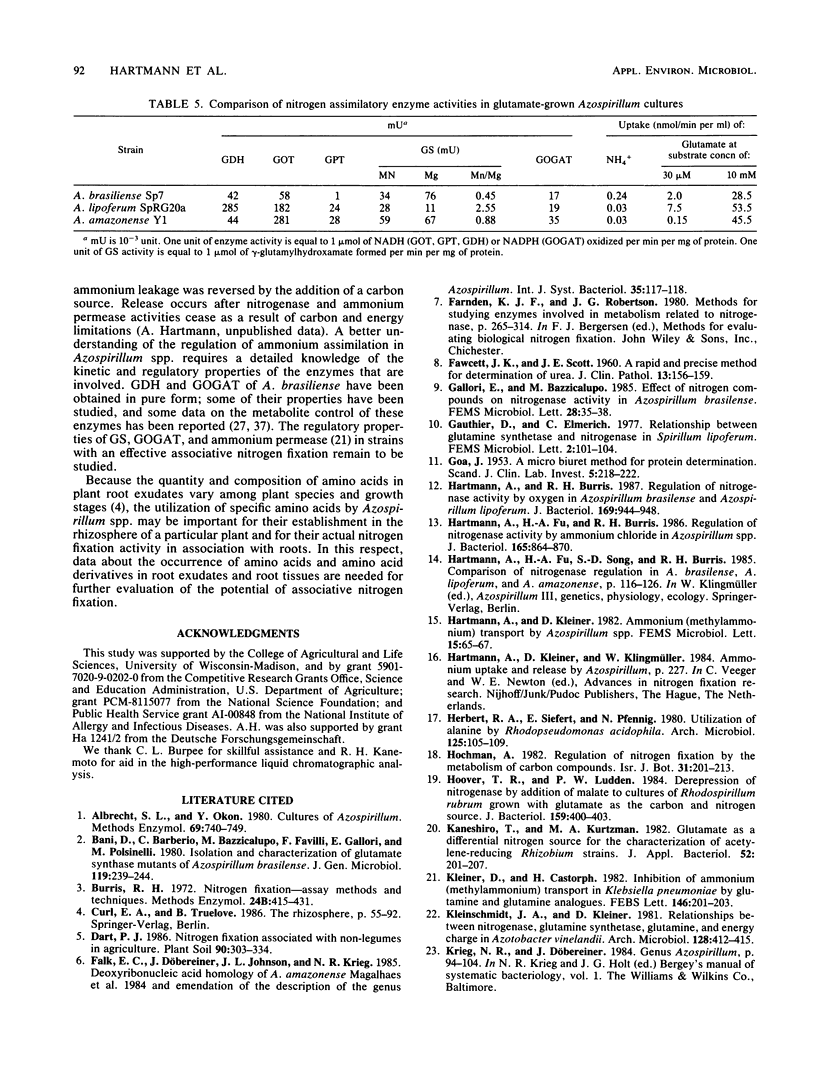
The utilization of amino acids for growth and their effects on nitrogen fixation differ greatly among the several strains of each species of Azospirillum spp. that were examined. A. brasiliense grew poorly or not at all on glutamate, aspartate, serine, or histidine as the sole nitrogen and carbon sources. Nitrogen fixation by most A. brasiliense strains was inhibited only slightly even by 10 mM concentrations of these amino acids. In contrast, A. lipoferum and A. amazonense grew very well on glutamate, aspartate, serine, or histidine as the sole nitrogen and carbon sources; nitrogen fixation, which was measured in the presence of malate or sucrose, was severely inhibited by these amino acids. It was concluded that growth on histidine as the sole source of nitrogen, carbon, and energy may be used for the taxonomic characterization of Azospirillum spp. and for the selective isolation of A. lipoferum. The different utilization of various amino acids by Azospirillum spp. may be important for their establishment in the rhizosphere and for their associative nitrogen fixation with plants. The physiological basis for the different utilization of glutamate by Azospirillum spp. was investigated further. A. brasiliense and A. lipoferum exhibited a high affinity for glutamate uptake (Km values for uptake were 8 and 40 microM, respectively); the Vmax was 6 times higher in A. lipoferum than in A. brasiliense. At high substrate concentrations (10 mM), the nonsaturable component of glutamate uptake was most active in A. lipoferum and A. amazonense.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burris R. H. Nitrogen fixation--assay methods and techniques. Methods Enzymol. 1972;24:415–431. doi: 10.1016/0076-6879(72)24088-5. [DOI] [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Burris R. H. Regulation of nitrogenase activity by oxygen in Azospirillum brasilense and Azospirillum lipoferum. J Bacteriol. 1987 Mar;169(3):944–948. doi: 10.1128/jb.169.3.944-948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A., Fu H., Burris R. H. Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol. 1986 Mar;165(3):864–870. doi: 10.1128/jb.165.3.864-870.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Ludden P. W. Derepression of nitrogenase by addition of malate to cultures of Rhodospirillum rubrum grown with glutamate as the carbon and nitrogen source. J Bacteriol. 1984 Jul;159(1):400–403. doi: 10.1128/jb.159.1.400-403.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Castorph H. Inhibition of ammonium (methylammonium) transport in Klebsiella pneumoniae by glutamine and glutamine analogues. FEBS Lett. 1982 Sep 6;146(1):201–203. doi: 10.1016/0014-5793(82)80735-7. [DOI] [PubMed] [Google Scholar]
- Martinez-Drets G., Del Gallo M., Burpee C., Burris R. H. Catabolism of carbohydrates and organic acids and expression of nitrogenase by azospirilla. J Bacteriol. 1984 Jul;159(1):80–85. doi: 10.1128/jb.159.1.80-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik P., Ghosh S. NADPH/NADH-dependent cold-labile glutamate dehydrogenase in Azospirillum brasilense. Purification and properties. Eur J Biochem. 1986 Mar 17;155(3):595–602. doi: 10.1111/j.1432-1033.1986.tb09530.x. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- O'Gara F., Shanmugam K. T. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim Biophys Acta. 1976 Jul 21;437(2):313–321. doi: 10.1016/0304-4165(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Carbon and ammonia metabolism of Spirillum lipoferum. J Bacteriol. 1976 Nov;128(2):592–597. doi: 10.1128/jb.128.2.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Houchins J. P., Albrecht S. L., Burris R. H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977 Jan;98(1):87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- Ratti S., Curti B., Zanetti G., Galli E. Purification and characterization of glutamate synthase from Azospirillum brasilense. J Bacteriol. 1985 Aug;163(2):724–729. doi: 10.1128/jb.163.2.724-729.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibach P. H., Streeter J. G. Evaluation of active versus passive uptake of metabolites by Rhizobium japonicum bacteroids. J Bacteriol. 1984 Jul;159(1):47–52. doi: 10.1128/jb.159.1.47-52.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985 Apr;162(1):190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam K. T., Morandi C. Amino acids as repressors of nitrogenase biosynthesis in Klebsiella pneumoniae. Biochim Biophys Acta. 1976 Jul 21;437(2):322–332. doi: 10.1016/0304-4165(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Song S. D., Hartmann A., Burris R. H. Purification and properties of the nitrogenase of Azospirillum amazonense. J Bacteriol. 1985 Dec;164(3):1271–1277. doi: 10.1128/jb.164.3.1271-1277.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrand J. J., Krieg N. R., Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978 Aug;24(8):967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- Tubb R. S. Regulation of nitrogen fixation in Rhizobium sp. Appl Environ Microbiol. 1976 Oct;32(4):483–488. doi: 10.1128/aem.32.4.483-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Pengra R. M. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae. J Bacteriol. 1966 Sep;92(3):618–622. doi: 10.1128/jb.92.3.618-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


