Abstract
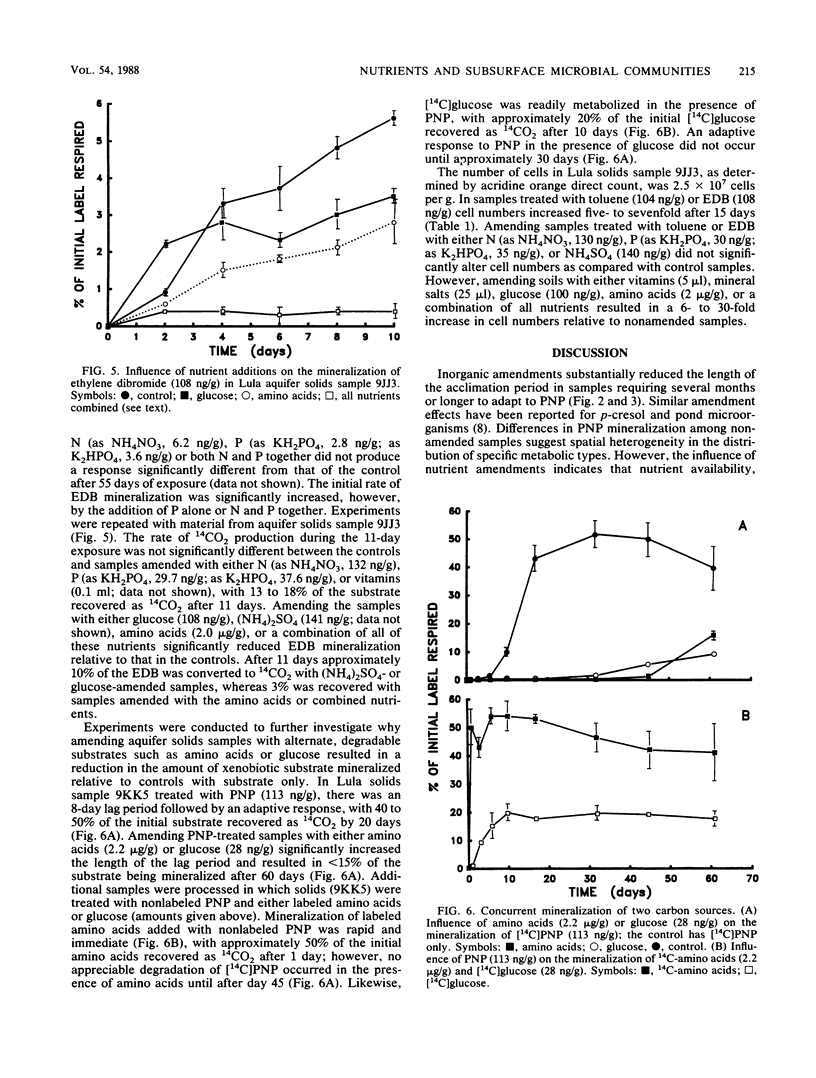
The influence of inorganic and organic amendments on the mineralization of ethylene dibromide, p-nitrophenol, phenol, and toluene was examined in subsurface soil samples from a pristine aquifer near Lula, Okla. The responses indicate that the metabolic abilities and nutrient requirements of groundwater microorganisms vary substantially within an aquifer. In some samples, additions of inorganic nutrients resulted in a more rapid adaptation to the test substrate and a higher rate of metabolism, indicating that metabolism may have been limited by these nutrients. In other samples from the same aquifer layer, inorganic amendments had little or no influence on mineralization. In general, the addition of multiple inorganic nutrients resulted in a greater enhancement of degradation than did the addition of single substances. Additions of alternate carbon sources, such as glucose or amino acids, inhibited the mineralization of the xenobiotic substrates. This inhibition appears to be the result of the preferential utilization of the more easily degradable carbon amendments.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aelion C. M., Swindoll C. M., Pfaender F. K. Adaptation to and biodegradation of xenobiotic compounds by microbial communities from a pristine aquifer. Appl Environ Microbiol. 1987 Sep;53(9):2212–2217. doi: 10.1128/aem.53.9.2212-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle T. W., Hullar M. A., Livingston R. J., Meeter D. A., White D. C. Spatial distribution of biochemical parameters indicating biomass and community composition of microbial assemblies in estuarine mud flat sediments. Appl Environ Microbiol. 1983 Jan;45(1):58–63. doi: 10.1128/aem.45.1.58-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Kollig H. P., Hodson R. E. Nutrient limitation and adaptation of microbial populations to chemical transformations. Appl Environ Microbiol. 1986 Mar;51(3):598–603. doi: 10.1128/aem.51.3.598-603.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


