Abstract
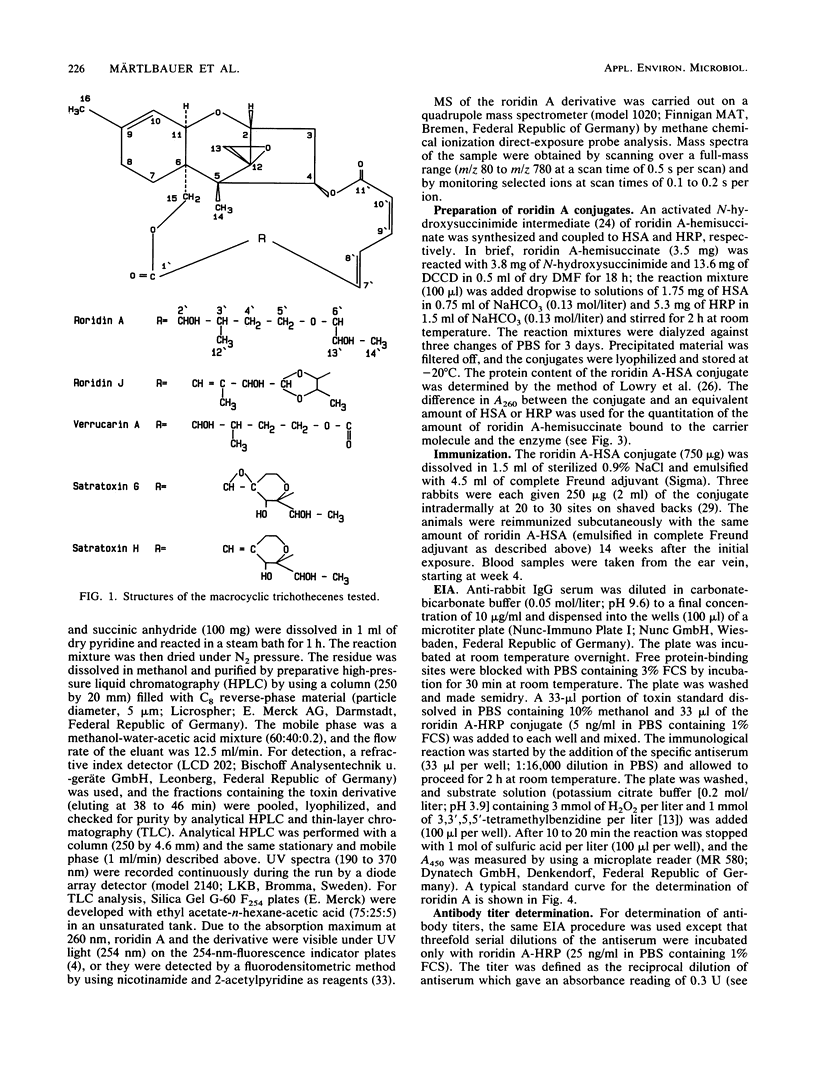
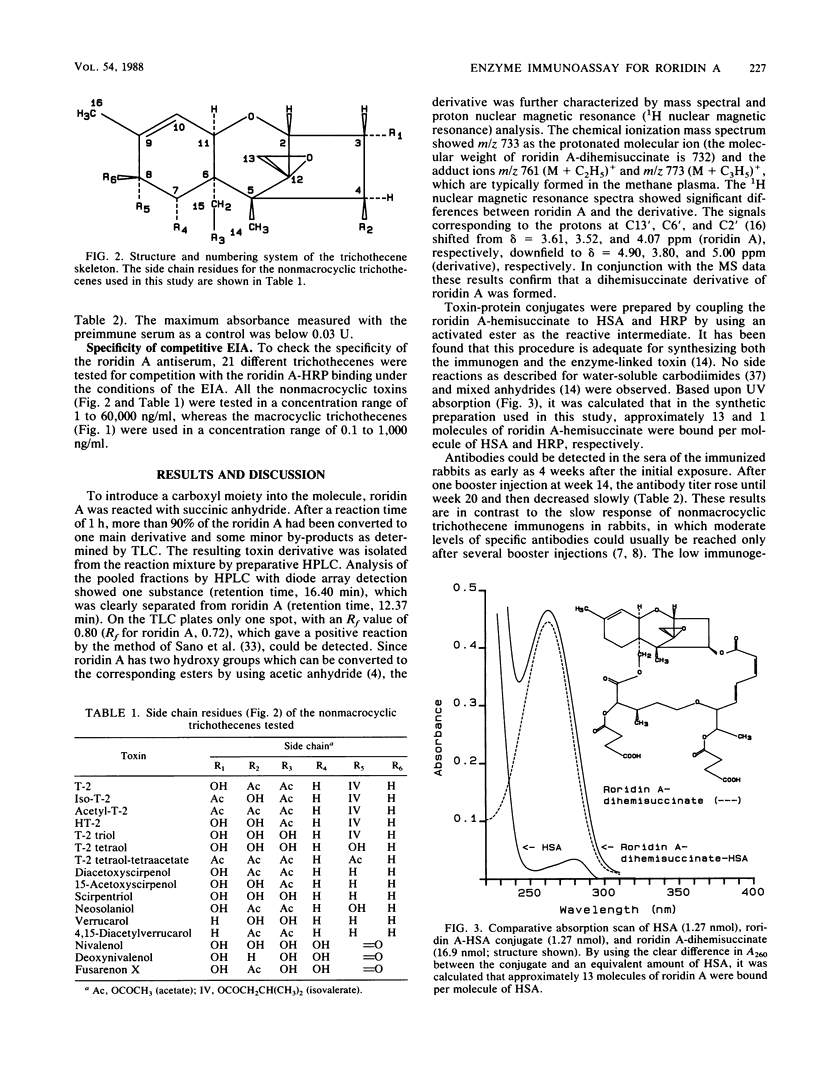
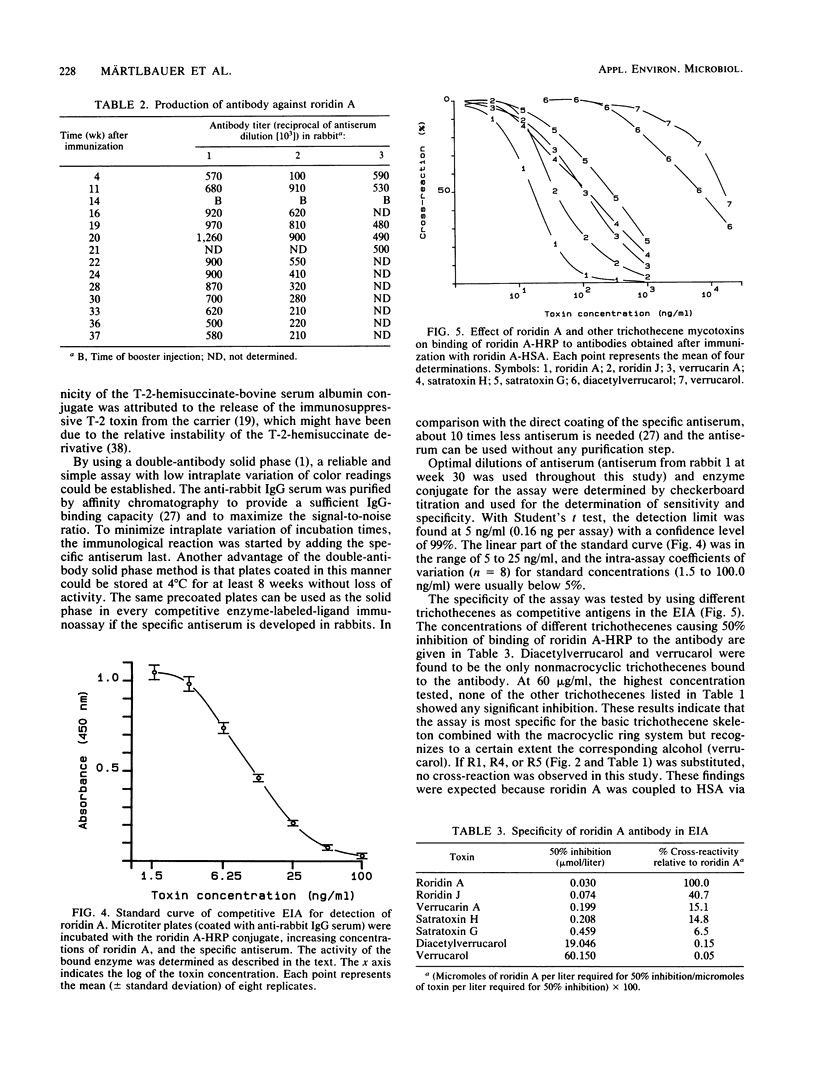
Antisera against roridin A were prepared by using a roridin A-hemisuccinate derivative coupled to human serum albumin as the immunogen. Antibodies could be detected in the sera of the immunized rabbits as early as 4 weeks after the initial exposure. After one booster injection at week 14, high antibody titers were measured over a period of 21 weeks. The specificity and sensitivity of the antibodies were tested by using roridin A-hemisuccinate coupled to horseradish peroxidase as an enzyme-linked toxin in a competitive assay with a double-antibody solid phase. The assay was most specific for the tested macrocyclic trichothecenes, and the relative cross-reactivities with roridin A, roridin J, verrucarin A, satratoxin H, and satratoxin G were 1, 0.41, 0.15, 0.15, and 0.07, respectively. When 16 nonmacrocyclic trichothecenes were tested, only diacetylverrucarol (0.0015) and verrucarol (0.0005) showed minor cross-reactivity. The sensitivity of the enzyme immunoassay for the detection of roridin A was in the range of 5 to 50 ng/ml (0.16 to 1.6 ng per assay).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa H., Maeda M., Tsuji A., Kambegawa A. Chemiluminescence enzyme immunoassay of dehydroepiandrosterone and its sulfate using peroxidase as label. Steroids. 1981 Oct;38(4):453–464. doi: 10.1016/0039-128x(81)90079-9. [DOI] [PubMed] [Google Scholar]
- Bata A., Harrach B., Ujszászi K., Kis-Tamás A., Lásztity R. Macrocyclic trichothecene toxins produced by Stachybotrys atra strains isolated in Middle Europe. Appl Environ Microbiol. 1985 Mar;49(3):678–681. doi: 10.1128/aem.49.3.678-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busam L., Habermehl G. G. Accumulation of mycotoxins by Baccharis coridifolia: a reason for livestock poisoning. Naturwissenschaften. 1982 Aug;69(8):392–393. doi: 10.1007/BF00396694. [DOI] [PubMed] [Google Scholar]
- Böhner B., Tamm C. Die Konstitution von Roridin A. Verrucarine und Roridine, 11. Helv Chim Acta. 1966 Dec 10;49(8):2527–2546. doi: 10.1002/hlca.19660490817. [DOI] [PubMed] [Google Scholar]
- Chu F. S., Grossman S., Wei R. D., Mirocha C. J. Production of antibody against T-2 toxin. Appl Environ Microbiol. 1979 Jan;37(1):104–108. doi: 10.1128/aem.37.1.104-108.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. S., Liang M. Y., Zhang G. S. Production and characterization of antibody against diacetoxyscirpenol. Appl Environ Microbiol. 1984 Oct;48(4):777–780. doi: 10.1128/aem.48.4.777-780.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. S., Zhang G. S., Williams M. D., Jarvis B. B. Production and characterization of antibody against deoxyverrucarol. Appl Environ Microbiol. 1984 Oct;48(4):781–784. doi: 10.1128/aem.48.4.781-784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallati H., Pracht I. Peroxidase aus Meerrettich: Kinetische Studien und Optimierung der Peroxidase-Aktivitätsbestimmung mit den Substraten H202 und 3,3',5,5'-Tetramethylbenzidin. J Clin Chem Clin Biochem. 1985 Aug;23(8):453–460. [PubMed] [Google Scholar]
- Gendloff E. H., Casale W. L., Ram B. P., Tai J. H., Pestka J. J., Hart L. P. Hapten-protein conjugates prepared by the mixed anhydride method. Cross-reactive antibodies in heterologous antisera. J Immunol Methods. 1986 Aug 21;92(1):15–20. doi: 10.1016/0022-1759(86)90497-7. [DOI] [PubMed] [Google Scholar]
- Gendloff E. H., Pestka J. J., Swanson S. P., Hart L. P. Detection of T-2 toxin in Fusarium sporotrichioides-infected corn by enzyme-linked immunosorbent assay. Appl Environ Microbiol. 1984 May;47(5):1161–1163. doi: 10.1128/aem.47.5.1161-1163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermehl G. G., Busam L., Heydel P., Mebs D., Tokarnia C. H., Döbereiner J., Spraul M. Macrocyclic trichothecenes: cause of livestock poisoning by the Brazilian plant Baccharis coridifolia. Toxicon. 1985;23(5):731–745. doi: 10.1016/0041-0101(85)90003-0. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Brimfield A. A., Miller M., Finkelman F. D., Chu S. F. Preparation and characterization of monoclonal antibodies to the trichothecene mycotoxin T-2. Appl Environ Microbiol. 1985 Jan;49(1):168–172. doi: 10.1128/aem.49.1.168-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. B., Midiwo J. O., Tuthill D., Bean G. A. Interaction Between the Antibiotic Trichothecenes and the Higher Plant Baccharis megapotamica. Science. 1981 Oct 23;214(4519):460–462. doi: 10.1126/science.214.4519.460. [DOI] [PubMed] [Google Scholar]
- Kohen F., Pazzagli M., Kim J. B., Lindner H. R. An immunoassay for plasma cortisol based on chemiluminescence. Steroids. 1980 Oct;36(4):421–437. doi: 10.1016/0039-128x(80)90030-6. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy T., Sarver E. W., Greene S. L., Jarvis B. B. Mass spectral investigations on trichothecene mycotoxins. II. Detection and quantitation of macrocyclic trichothecenes by gas chromatography/negative ion chemical ionization mass spectrometry. J Assoc Off Anal Chem. 1987 Jan-Feb;70(1):132–140. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mortimer P. H., Campbell J., Di Menna M. E., White E. P. Experimental myrotheciotoxicosis and poisoning in ruminants by verrucarin A and roridin A. Res Vet Sci. 1971 Nov;12(6):508–515. [PubMed] [Google Scholar]
- Peters H., Dierich M. P., Dose K. Enzyme-linked immunosorbent assay for detection of T-2 toxin. Hoppe Seylers Z Physiol Chem. 1982 Dec;363(12):1437–1441. doi: 10.1515/bchm2.1982.363.2.1437. [DOI] [PubMed] [Google Scholar]
- Samples D., Hill D. W., Bridges C. H., Camp B. J. Isolation of a mycotoxin (roridin A) from Phomopsis spp. Vet Hum Toxicol. 1984 Feb;26(1):21–23. [PubMed] [Google Scholar]
- Scott P. M. Assessment of quantitative methods for determination of trichothecenes in grains and grain products. J Assoc Off Anal Chem. 1982 Jul;65(4):876–883. [PubMed] [Google Scholar]
- Smith R. D., Udseth H. R., Wright B. W. Rapid and high resolution capillary supercritical fluid chromatography (SFC) and SFC/MS of trichothecene mycotoxins. J Chromatogr Sci. 1985 May;23(5):192–199. doi: 10.1093/chromsci/23.5.192. [DOI] [PubMed] [Google Scholar]
- Wood W. G. Luminescence immunoassays: problems and possibilities. J Clin Chem Clin Biochem. 1984 Dec;22(12):905–918. [PubMed] [Google Scholar]
- Zhang G. S., Schubring S. L., Chu F. S. Improved method for production of antibodies against T-2 toxin and diacetoxyscirpenol in rabbits. Appl Environ Microbiol. 1986 Jan;51(1):132–137. doi: 10.1128/aem.51.1.132-137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



