Abstract
Here, we show that bacteria induce de novo synthesis of both major histocompatability complex (MHC) class I and II molecules in a mouse dendritic cell culture system. The neo-biosynthesis of MHC class I molecules is delayed as compared with that of MHC class II. Furthermore, bacteria stabilize MHC class I molecules by a 3-fold increase of their half-life. This has important consequences for the capacity of dendritic cells to present bacterial antigens in the draining lymph nodes. In addition, a model antigen, ovalbumin, expressed on the surface of recombinant Streptococcus gordonii is processed and presented on MHC class I molecules. This presentation is 106 times more efficient than that of soluble OVA protein. This exogenous pathway of MHC class I presentation is transporter associated with antigen processing (TAP)-dependent, indicating that there is a transport from phagolysosome to cytosol in dendritic cells. Thus, bacteria are shown to be a potentially useful mean for the correct delivery of exogenous antigens to be presented efficiently on MHC class I molecules.
Dendritic cells (DC) are antigen-presenting cells, which are crucial for generating primary T cell immune responses (1). They are particularly distributed in tissues that provide an environmental interface (the skin and mucosal surfaces) and in lymphoid organs (2), where they act as sentinels for incoming pathogens. Inflammatory signals [tumor necrosis factor (TNF)-α and interleukin (IL)-1β] as well as bacterial products [lipopolysaccharide (LPS) and lipoteichoic acid] induce migration of antigen-loaded DC from peripheral tissues to secondary lymphoid organs (3, 4). During this migration, DC mature and up-regulate surface major histocompatability complex (MHC) II molecules and costimulatory molecules, thus augmenting their ability to prime T cells. Peptide-pulsed or viral particle-pulsed DC can trigger both CD4+ and CD8+ T cell responses in vivo (5–11).
We have previously shown that, similarly to macrophages (12–16), cloned DC can efficiently process exogenous viral proteins for class I presentation to cytotoxic T cells (11). However, soluble proteins are very poorly presented on MHC class I molecules through a mechanism that is TAP dependent (17). In contrast, particulate antigens, which enter the cells via phagocytosis, are processed and presented in association with MHC class I molecules with much greater efficiency via a mechanism that is still unknown (11, 18, 19). DC are essential for priming the immune system to antigens, and because they are present in tissues that interface the environment, they may encounter pathogens soon after invasion. In vitro infection of DC with bacteria results, indeed, in cell activation and in induced antigen-specific T cell proliferative responses (20–23).
We have previously described a DC culture system that has enabled us to culture, indefinitely, growth factor-dependent immature DC (24). These cells represent splenic myeloid DC because they are granulocyte/macrophage colony-stimulating factor dependent and lack the expression of CD8a. With this unique system, DC can be driven to full maturation by using different stimuli. In this study, we show that living bacteria induce de novo synthesis of both MHC class I and II molecules. Interestingly, the neo-biosynthesis of MHC class I molecules is delayed as compared with that of MHC class II. Furthermore, bacteria stabilize MHC class I molecules by a 3-fold increase of their half-life. In addition, a model antigen, ovalbumin, expressed on the surface of recombinant Streptococcus gordonii, is processed and presented on MHC class I molecules 106 times more efficiently than soluble OVA protein. This exogenous pathway of MHC class I presentation is TAP dependent, indicating that in DC there is a transport from phagolysosome to cytosol.
MATERIALS AND METHODS
DC and Culture Medium.
The D1 culture medium was IMDM (Sigma) containing 10% heat-inactivated fetal bovine serum (GIBCO), 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine (all from Sigma), and 50 μM 2-mercaptoethanol (complete IMDM) with 30% supernatant from R1 medium (24). Tap1−/− mice bred in a C57BL/6 background (25) were purchased at Centre de Developpement des Technique Avancées pour l’expérimentation animale. Tap1−/− DC were purified from bone marrow after 14 days of culture in 30% R1 medium by positive selection with N418 antibody (anti-CD11c) coupled to magnetic microbeads, using MiniMACS separation columns (all from Miltenyi Biotec, Auburn, CA). For bacteria-DC incubations, antibiotics were not added to the complete medium.
Antibodies and Cell Surface Phenotype.
The following monoclonal antibodies were purchased from PharMingen: H-2Db (28–14-8), CD80/B7.1 (1G10), CD40 (HM40–3), CD54/ICAM-1 (3E2), IA/Ed (2G9), CD86/B7.2 (GL1), VLA-4 (PS/2). Phycoerythrin-streptavidin was from Sigma. Hybridoma 2.4G2 (rat anti-mouse Fcγ II/III receptor, CD32) was a kind gift from B. Kyewski (DKFZ, Heidelberg, Germany). Before labeling experiments, FcR blocking was performed by incubating cells with 2.4G2 supernatant. Staining was carried out according to standard immunofluorescence techniques, and flow cytometry analysis was performed with a FACScan (Lysis II and Cell Quest software; Becton Dickinson).
Bacteria: Construction of Recombinant S. gordonii.
The recombinant strain of S. gordonii, expressing OVA, was constructed using the host-vector system GP1221-pSMB55, which has been described in detail (26) and named GP1252. OVA is expressed on the bacterial cell surface as a fusion with the M6 streptococcal, which is used as a fusion partner for surface expression of heterologous antigens in Gram-positive bacteria (27). The DNA encoding for 339 amino acids of OVA (amino acids 48–386, GenBank accession no. V00383) was obtained by PCR using primers CTAGATCTGACAGCACCAGGACAC and TAAAGCTTTAGGGGAAACACATCTG and cloned in the insertion vector pSMB55 cut with HindIII and BglII. Surface expression of the M6-OVA fusion protein in GP1252 was confirmed by immunofluorescence and Western blot of cell fractions, using M6- and ovalbumin-specific rabbit polyclonal antibodies (28).
Preparation of Bacteria for Assays with DC.
The wild-type S. gordonii strain GP204 (29) and the OVA-expressing recombinant GP1252 were grown at 37°C in tryptic soy broth without dextrose (Difco) and harvested by centrifugation at the end of the exponential phase of growth. Bacterial cells were then washed and resuspended in fresh medium containing 10% glycerol at 1:500 of the original culture volume. Aliquots were stored frozen at −70°C until use.
Cytokine Secretion.
D1 cells were incubated at 3 × 105 cells/ml in complete medium and incubated with wild-type S. gordonii GP204 at a ratio of 10 bacteria to 1 DC. Eighteen hours later, culture supernatants were collected and tested for cytokine production by ELISA. IL-1β and IL-6 were tested by using ELISA kits purchased from Genzyme. TNF-α was measured with Genzyme mouse TNF-α DuoSeT. IL-10 capture (JES5–2A5) and detection antibodies (biotinylated SXC-1) as well as recombinant IL-10 were from PharMingen and were used in sandwich ELISAs according to manufacturer’s instructions. IL-12 capture (α-IL12 p75; 9A5) and detection (α-IL12 p40; 5C3) antibodies as well as recombinant IL-12 were kindly provided by Dr. D. H. Presky (Hoffman–La-Roche).
Transmission Electron Microscopy.
For times varying between 30 min and 18 h, DC were incubated at 5 × 105 cells/ml in DC medium in suspension culture dishes (Corning) with live or heat-killed S. gordonii (GP204) at a ratio of 10 bacteria to 1 DC. A total of 5 × 105 cells were pelleted at 1200 rpm for 5 min, washed twice with PBS, and fixed in cold 2.5% glutaraldehyde for 4 h at 4°C. The specimens were prepared for electron microscopy according to standard protocols (30). In short, they were post-fixed with reduced osmium, encapsulated in agar, stained with uranyl acetate and phosphotungstic acid, and dehydrated in a series of graded ethanolic solutions finishing with pure acetone before finally being embedded in Epon 812. Ultrathin sections were cut and placed under 200 mesh standard square copper grids, contrasted with uranyl acetate and lead citrate, and examined with a Zeiss-type 906 transmission electron microscope.
MHC Class I and II Synthetic Rate.
DC were incubated for times varying between 1 and 36 h with S. gordonii GP204 at a ratio of 10 bacteria to 1 DC in IMDM with 10% fetal bovine serum (GIBCO). Cells were labeled with 1 mCi/ml [35S]methionine/cysteine (Promix, Amersham) for 30 min and then lysed. Lysates were precleared twice with Zysorbin (Zymed) and once with protein G-Sepharose (Pharmacia). MHC class I molecules were immunoprecipitated overnight using a monoclonal anti-class I 20.8.4S (ATCC) and protein G-Sepharose, whereas class II molecules were immunoprecipitated for 2 h with a rabbit polyclonal anti I-Aα antibody (made against a peptide corresponding to the entire cytoplasmic tail of I-Aα chain) and protein G-Sepharose. Immunoprecipitates were analyzed on 12% (class II) or 10% (class I) SDS/PAGE and quantified. Quantification was performed either with the imagequant software on a Molecular Dynamics Phosphorimager or with the bio-1d software (Vilber Lourmat, France) after scanning of the autoradiographs with a video camera.
Half-Lives of MHC Class I and II.
DC were incubated in IMDM with 10% fetal bovine serum (GIBCO) for 18 h in both the presence and absence of S. gordonii GP204 at a ratio of 10 bacteria to 1 DC. Cells were pulse-labeled for 30 min with 1 mCi/ml [35S]methionine/cysteine and then chased for different periods of time in the presence of an excess of cold methionine and in the absence of bacteria. The same amount of cells was lysed, and class I molecules were immunoprecipitated overnight with 20.8.4S-coated protein G-Sepharose, whereas mature class II molecules were immunoprecipitated for 2 h with Y3P monoclonal antibody-coated protein G-Sepharose. Immunoprecipitates were analyzed on 12% (class II) or 10% (class I) SDS/PAGE and quantified as above.
Antigen Presentation Assay.
D1 cells or Tap1−/− DC were seeded (1 × 104 cells/well) in flat-bottomed microtiter plates (Corning) pulsed with wild-type strain GP204, the OVA-expressing recombinant GP1252, ovalbumin, or SIINFEKL peptide for different times from 2 to 16 h in IMDM with 0.3% normal mouse serum supplemented with 2 mM l-glutamine and 50 μM 2-mercaptoethanol but without antibiotics. Plates were then washed twice with medium without FCS, and cells were fixed for 30 s on ice with 0.05% glutaraldehyde in medium. Unreacted glutaraldehyde was neutralized with 0.1 M PBS/glycine, and plates were washed for three times with IMDM with 5% FCS by centrifuging the plates and replacing the medium. Then, 5 × 104 B3Z (31) T cell hybridoma cells, which recognize the SIINFEKL/Kb complex, were seeded in a final volume of 200 μl of IMDM with 5% FCS supplemented with 50 μg/ml Gentamycin. IL-2 produced by T cell hybridoma was quantitated using IL-2-dependent cytotoxic T lymphocyte (CTL).
RESULTS
Immature Mouse Spleen DC Uptake Bacteria by Conventional Phagocytosis.
In this study, we have used the commensal bacterium S. gordonii because it is part of the normal microbial flora of the human oral cavity and has been successfully used as a vaccine vector for mucosal delivery of vaccine antigens (27). Indeed, recombinant strains of S. gordonii can colonize mucosal epithelia and induce both local and systemic antibody responses (32, 33). In addition to the conventional zipper-type phagocytosis, DC use coiling phagocytosis to internalize pathogens such as Borrelia burgdorferi (34). Coiling phagocytosis of bacteria delivers them into the cytosol, where bacterial antigens can join the endogenous route of MHC class I presentation (35, 36). To determine how DC internalize S. gordonii, DC were incubated with live and heat-killed bacteria at an estimated ratio of 10 bacteria to 1 DC.
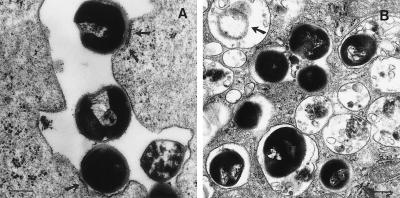
S. gordonii was internalized by DC using only conventional phagocytosis. Just 30 min after infection, some bacteria were contacting the cell membrane, inducing local thickening of plasma membrane (Fig. 1A); others had already been internalized, and at least 20 bacteria could be seen in large phagosomes. Four hours after infection, bacteria were found, in a partially degraded form, inside phagolysosomes (Fig. 1 B). Heat-killed bacteria were phagocytosed in a similar fashion (data not shown), thus indicating that internalization was not due to an active bacterial mechanism.
Figure 1.
DC internalize S. gordonii via conventional phagocytosis. DC were incubated with bacteria for different time periods at a ratio of 10 bacteria to 1 DC, then washed and processed for transmission electron microscopy. (A) Thirty minutes after infection, bacteria were contacting the cell membrane and inducing local thickening of plasma membrane (arrows) (magnification, ×39,000; bar represents 3.9 μm). (B) Four hours after infection, bacteria were found in phagolysosomes in a partially degraded form (arrow) at a magnification of ×28,700; bar = 2.9 μm.
Bacterial Uptake, but Not Latex Beads, Activates DC and Induces Cytokine Production.
We have previously shown that homogeneous immature growth-factor-dependent long-term DC line (D1) is induced to full maturation by different inflammatory stimuli such as TNF-α or IL-1β (24) and by bacterial cell products such as LPS or lipotheicholic acid.
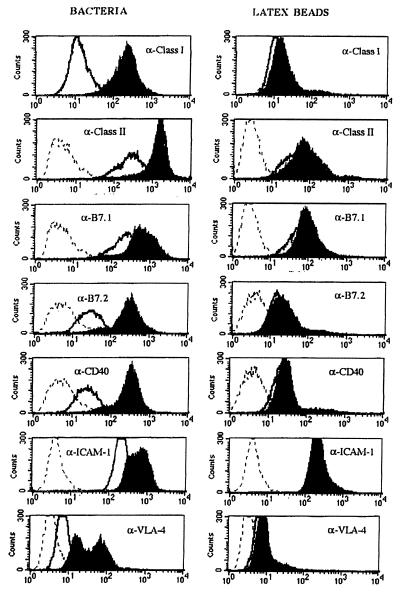
Here, we have analyzed phenotypical changes in terms of cell surface marker modulation with either bacteria or latex beads, both of which induce phagocytosis. D1 were incubated for 18 h with either 2-μm latex beads or S. gordonii at a ratio of 10 bacteria to 1 D1. Fig. 2 shows the FACS profiles of the analyzed cell surface molecules involved in DC activation and maturation. Clearly, only after incubation with bacteria, both MHC class I and II molecules and costimulatory molecules such as B7.1 (CD80), B7.2 (CD86), and CD40 were strongly up-regulated on the cell surface. The same feature was observed with adhesion molecules VLA-4 and ICAM-1 (CD54) (Fig. 2).
Figure 2.
Phenotypical maturation of D1 cells. FACS profiles of surface markers after incubation with S. gordonii at a ratio of 10 bacteria to 1 DC (Left) or latex beads at a ratio of 100 beads to 1 DC (Right). Histograms represent the following: filled, cells after treatment; open, untreated cells; dashed, isotype controls.
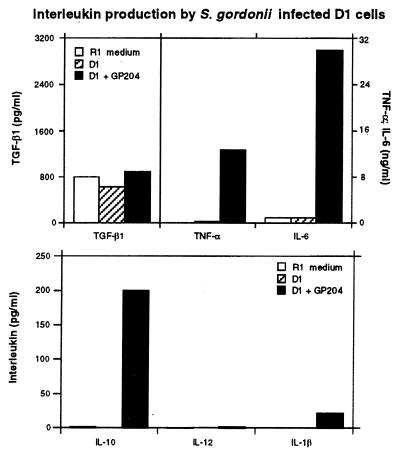
To test whether the observed phenotypical maturation was correlated to a certain pattern of cytokine production, we measured cytokine release in culture supernatant by ELISA (Fig. 3). Bacteria-treated D1 cells produced a large amount of TNF-α and IL-6 (12 and 30 ng/ml, respectively) but only a limited amount of IL-10 (0.2 ng/ml) and IL-1β (50 pg/ml). IL-12 p75 level was below the detection limits of 10 pg/ml; this was expected as we and others have previously shown that DC do not produce significant amounts of IL-12 unless triggered by MHC class II and CD40 engagement or during T cell antigen presentation (24, 37, 38). To rule out the possibility that DC maturation could have been induced through an autocrine amplification of cytokine production, D1 cells were incubated with bacteria in the presence of neutralizing anti-TNF-α antibody. Because IL-6 did not induce DC maturation (24), anti-IL-6 antibodies were not added. D1 maturation could be only partly inhibited by anti-TNF-α antibodies, suggesting that other additional mechanisms are involved in bacteria-induced maturation (data not shown). Thus, bacteria can induce DC activation through a still unknown mechanism that does not operate via TNF-α and that may be mediated by membrane receptors.
Figure 3.
S. gordonii-treated DC secrete large amounts of inflammatory cytokines. Culture supernatants from untreated cells (hatched bars), S. gordonii-treated D1 (black bars), or R1 culture medium (white bars) were tested for cytokine release by ELISA. Recombinant cytokines were used as standards in each ELISA.
Bacterial Uptake Up-Regulates the Synthesis of MHC Class I Molecules and Enhances Their Half-Life.
We have shown that bacteria induce up-regulation of cell surface MHC I molecules. Thereafter, we analyzed whether bacteria-induced maturation of D1 cells had any effect on MHC class I synthesis and stability.
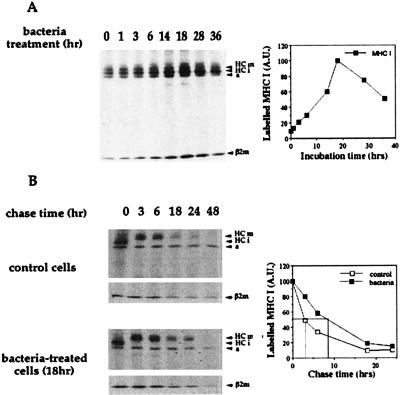
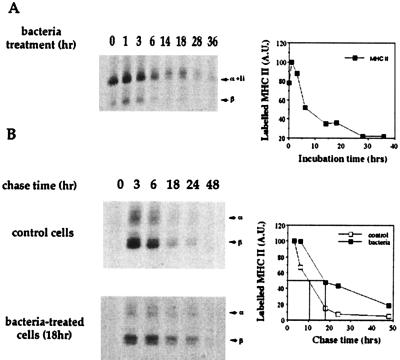
D1 cells were incubated for periods of time between 1 and 36 h with S. gordonii wild-type GP204 at a ratio of 10 bacteria to 1 DC. Cells were then pulse-labeled with [35S]methionine/cysteine. As shown in Fig. 4A, MHC class I synthesis was induced very slowly, reaching a peak only after 18 h of activation. The synthesis was then sustained for several hours. The half-life of newly synthesized MHC class I molecules was also analyzed so as to correlate it with the maturation process. DC were activated by bacteria for 18 h, which corresponded to the time of maximum synthesis of class I molecules. Then, they were pulse-labeled with [35S]methionine/cysteine and chased in an excess of cold methionine, in the absence of the activation stimulus to mimic the physiological milieu in place when DC have left the inflamed site. Newly synthesized MHC class I molecules in immature DC had a very short (3 h) half-life; however, a 3-fold increase was observed after bacterial activation (Fig. 4B). This feature is in contrast with a previous report on human monocyte-derived DC (39). Inflammatory stimuli, such as LPS, were shown to induce up-regulation of MHC class I and II synthesis, but class I molecule stabilization was not observed in human DC (39).
Figure 4.
Bacterial infection induces de novo synthesis and stabilization of MHC class I molecules. (A) DC were metabolically labeled before (t = 0) or at different times (t = 1–36 h) after bacterial infection. HCm, heavy chain mature form; HCi, heavy chain immature form; a, actin; β2 m, β2 microglobulin. The graph shows the quantification by Phosphorimager of the radioactivity associated with MHC I heavy chain. A.U., arbitrary units. (B) Stability of MHC I molecules synthesized by untreated cells (Top) or by cells treated for 18 h with bacteria (Bottom). Cells were biosynthetically labeled and chased for 0–48 h in an excess of cold methionine. Abbreviations are as in A. The graph represents the quantification by bio-1d software of the radioactivity associated with MHC I molecules in treated and untreated DC chased in the absence of bacteria.
This is evidence for stabilization of MHC class I molecules in DC after activation with bacteria. Interestingly, bacterial activation did not change the rate of MHC class I maturation because the rate of appearance of an endoglycosidase H-resistant mature form was the same whether bacteria were present or absent (data not shown).
The Half-Life of MHC II Molecules Is Increased after Bacterial Encounter.
We and others have shown that in vitro maturation of mouse DC leads to up-regulation of MHC class II expression (24) and to the enhancement of MHC class II half-life (40). As expected, bacteria also induced up-regulation of MHC class II synthesis much more rapidly than with class I. One hour after bacterial infection, biosynthesis was already maximal, then it gradually decreased in nearly 20 h (Fig. 5A). Further, in non-activated cells, newly synthesized MHC class II molecules had a half-life of about 10 h, and this was increased 2-fold after bacterial activation (Fig. 5B). This result confirms that stimuli other than spontaneous in vitro activation can stabilize MHC class II molecules in mouse DC (40).
Figure 5.
Bacterial infection induces de novo synthesis and stabilization of MHC class II molecules. (A) DC were metabolically labeled before (t = 0) or at different times (t = 1–36 h) after bacterial infection. α + Ii, alpha chain + invariant chain; β, beta chain. The graph shows the quantification by Phosphorimager of the radioactivity associated with the α-chains. (B) Stability of mature MHC class II molecules synthesized by untreated cells (Top) or cells treated for 18 h with bacteria (Bottom). α, alpha chain; β, beta chain. The graph represents the quantification by bio-1d software of the radioactivity associated with β-chains in DC incubated or not with bacteria and then chased in the absence of bacteria.
Foreign Proteins Expressed by Bacteria Can Be Processed and Presented on MHC Class I Molecules by D1 Cells with Very High Efficiency As Compared with Soluble Antigens Delivered Through the Exogenous Pathway.
DC were incubated with wild-type S. gordonii (GP204) or with OVA-expressing recombinant GP1252. It was determined by Western blot analysis that each bacterium expressed an average of 1000 molecules of OVA on the cell surface (not shown).
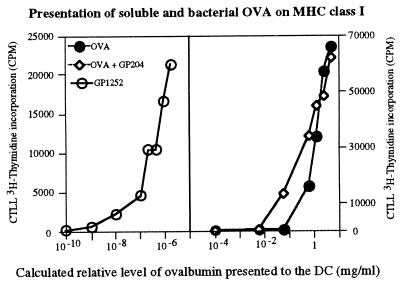
DC could process and present very efficiently bacterial antigens on MHC class I as measured by IL-2 production during antigen presentation using the B3Z hybridoma, which is specific for the SIINFEKL peptide in association with H-2Kb (Fig. 6). This process required at least 8 h because fixation of the antigen-presenting cells before 8 h resulted in no antigen presentation (data not shown). We compared the efficiency of class I MHC presentation of bacterial OVA with presentation of soluble OVA (Fig. 6). Clearly, soluble antigens are poorly presented on MHC class I, whereas a 106-fold increase in presentation was observed by using OVA-expressing S. gordonii (Fig. 6). This increase is not due solely to a bacterial adjuvant effect on DC activation because in the presence of wild-type bacteria, presentation of soluble OVA was increased only 10 times. Thus, the use of bacteria as vectors for antigen delivery resulted in the correct processing and presentation of the recombinant protein on MHC class I molecules with high efficiency.
Figure 6.
DC can process bacterial antigens for MHC I presentation very efficiently. DC were incubated with recombinant OVA-gordonii (GP1252, ○), wild-type gordonii (GP204) + exogenous OVA (◊), or OVA alone (•) for 16 h and then washed and fixed. The ratio of bacteria to DC ranges from 500:1 to 0.3:1. Ratio of 500 bacteria to 1 DC corresponds to 1 μg/ml of OVA. IL-2 production by B3Z T cell hybridoma was measured as [3H]thymidine incorporation by IL-2-dependent CTL.
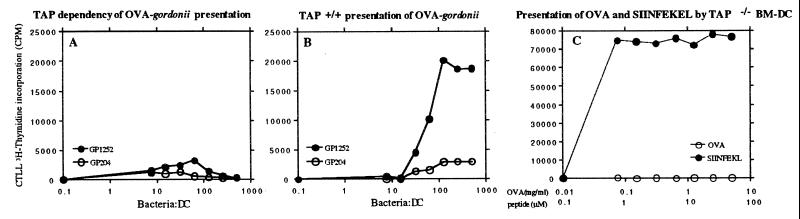
MHC I Presentation of Bacterial Proteins is TAP-Dependent.
To investigate whether loading of bacterial OVA occurred via the classical pathway of MHC class I loading (i.e., in a TAP-dependent manner) or via a non-cytosolic pathway (which is mostly TAP independent), we incubated D1 or TAP−/− BM-derived DC with either wild-type or OVA recombinant S. gordonii. As shown in Fig. 7 A and B, presentation of bacterial protein was completely abolished. As a control, the capacity of TAP−/− BM-derived DC to present exogenously generated peptide or soluble OVA was also measured (Fig. 7C).
Figure 7.
MHC class I presentation of bacterial antigens is TAP dependent. The B3Z hybridoma response to BM-derived TAP−/− DC (A and C) or to D1 TAP+/+ (B) incubated with the following is shown: (A and B) OVA-gordonii (GP1252, •) or wild-type gordonii (GP204, ○); (C) soluble OVA (○) or SIINFEKL peptide. (•).
DISCUSSION
Immature DC accomplish a sentinel function by sampling and processing encountered antigens and by loading them on MHC molecules (2). Inflammatory mediators can then induce a maturation process that triggers migration of DC to secondary lymphoid organs, where they can present previously captured and processed antigens and prime T cells (3). The molecular mechanisms leading to the “presenting” DC phenotype are mostly unknown. It has already been shown that the form of antigen is crucial for determining the efficiency of presentation on MHC class I and class II. The particulate form, which enters the cells via phagocytosis, has been identified as that which most powerfully increases antigen presentation function (11, 18, 19). In this study, we have compared the capacity of different phagocytosed material to activate DC. We took advantage of a DC culture system, developed in our laboratory (24), that enabled us to grow mouse spleen immature DC. This unique culture system has proven to be very useful for studying in vitro DC maturation (24).
Here, we have shown that phagocytosis per se is not sufficient to activate DC and that latex beads, which are rapidly phagocytosed by DC, are not able to induce DC maturation. On the other hand, bacteria are internalized very efficiently by DC, and they induce both phenotypical and functional maturation. This was shown by the production of inflammatory cytokines, such as TNF-α and IL-6 and by up-regulation of MHC and costimulatory molecules. TNF-α production after bacterial infection was not responsible for the complete activation of DC through an autocrine loop, as neutralizing antibodies only partly inhibited DC maturation. A previous report on human DC has also shown that Mycobacterium tubercolosis can induce phenotypical maturation of DC, although in this case HLA-DR and CD86 molecules were already very highly expressed, and further modulations could not be detected (20). This suggested that these DC were not completely immature.
Bacterial activation induces transient de novo synthesis of MHC class I molecules and an increase of their stability. The synthesis of MHC class I molecules is delayed when compared with that of class II molecules. This could be explained by the need to reduce the chances of presenting self-peptides in association with MHC class I during a bacterial infection. Processing and presentation of the bacterial antigens requires several hours, and 8 h were estimated to be necessary for the processing and presentation of antigens derived from S. gordonii. Thus, delaying MHC class I synthesis so that it begins very slowly and reaches a peak at 18 h, which coincides with maximal DC activation, would increase the chances of presenting mostly bacteria-derived peptides. Moreover, the half-life of newly synthesized MHC class I molecules is increased 3-fold, changing from 3 to 9 h. This is particularly relevant in view of the recent finding that peptide-pulsed DC were detected in the draining lymph node 8 h after subcutaneous injection reaching a peak level at 24 h and then declining at 48 h when antigen-specific T cells were present (41). This suggests that after T cell priming, DC are rapidly cleared from the lymph node. Thus, stabilization of MHC class I molecules resulting in a half-life of 9 h would guarantee that antigen-loaded DC, which have abandoned the inflamed site to migrate to the lymph node, could accomplish their final task of presenting, to naive CTLs, mostly peptides derived from the processing of proteins of the danger signal. Nevertheless, stabilization of MHC class I molecules could not lead to everlasting MHC–peptide complexes because DC should not be killed by activated CTLs, and this would explain their still limited half-life. In contrast, the stability of MHC class I molecules in human (39) was not changed when DC were treated with bacterial products, such as LPS.
Regarding MHC class II molecules, up-regulation of synthesis is very rapid, reflecting the capacity of DC to present exogenous captured antigens to CD4 T cells only a few hours after antigen internalization (39, 42). Bacteria also induce increased stability of MHC class II molecules, which is in absolute numbers much higher than that of class I molecules (17 h instead of 9 h). The high stability of class II molecules as well as the existence of antigen retention compartments, recently described for immature DC (42), could account for the ability of DC to present antigens on MHC class II long after antigen exposure (39, 40). In contrast, MHC class I–peptide complexes are rapidly degraded. Indeed, MHC class I antigen presentation was undetectable 48 h after antigen encounter (data not shown).
Bacteria induce a rapid maturation of DC, and this correlates to the capacity of DC to present antigens on MHC molecules. The model antigen OVA is presented through the exogenous pathway of MHC class I presentation with a 106-fold higher efficiency, when expressed on the bacterial wall as compared with soluble OVA. DC have been already shown to process and present defined epitopes expressed as bacterial cytoplasmic fusion proteins (23). However, up to now the efficiency of presentation has not been compared with that of the soluble protein. In this report, we have described the highest efficiency of presentation of exogenous proteins on MHC I, higher than that described for OVA coupled to beads (18). This is not due solely to the capacity of bacteria to induce DC maturation because the co-incubation of wild-type sgordonii and soluble OVA increased antigen presentation efficiency by only 10-fold, although one should consider that with primary T cells that are more sensitive to costimulation, there may be some differences. Taken as a whole, these results indicate that for a rational design of new vaccines aimed to stimulate dendritic cells, bacteria are one of the best candidates as vectors.
Two mechanisms of exogenous antigen loading of MHC class I molecules have been described so far. In one pathway, exogenous antigens gain access to the cytosol and are presented in a TAP-dependent manner (12, 13, 14, 17). The other pathway is non-cytosolic, and presentation is mostly TAP independent (16, 43). In our study, we have shown that bacterial OVA is processed via a TAP-dependent mechanism, indicating a classical loading of MHC class I molecules. Acidic compartments are required for processing of bacterial proteins (23), indicating that the phagolysosome is an obligatory route. The molecular nature of putative phagolysosome–cytosol transporters remains to be elucidated.
Acknowledgments
We thank Dr. Paolo Dellabona for providing us with the TAP1−/− mice with permission from Dr. S. Tonegawa, Renato Longhi for synthesizing the SIINFEKL peptide, and MILTENYI Biotec GmbH for kindly providing the anti-mouse CD11c (N418) antibody coupled to magnetic microbeads. This study was supported by grants from Consiglio Nazionale delle Ricerche, EC-TMR network Contract FMRX-CT96-0053; BIOTECH Programme (contract no. BIO4-CT96-0452), and the Istituto Superiore di Sanità (Research project 52 on MS and AIDS) and BIOTOP.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: DC, dendritic cells; LPS, lipopolysaccharide; OVA, ovalbumin; MHC, major histocompatability complex; TNF, tumor necrosis factor; IL, interleukin; CTL, cytotoxic T lymphocyte; TAP, transporter associated with antigen processing.
References
- 1.Steinman R M. Ann Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Austyn J M. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roake J A, Rao A S, Morris P J, Larsen C P, Hankins D F, Austyn J M. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cumbertach M, Kimber I. Immunology. 1992;75:257. [PMC free article] [PubMed] [Google Scholar]
- 5.Paglia P, Girolomoni G, Robbiati F, Granucci F, Ricciardi-Castagnoli P. J Exp Med. 1993;178:1893–1901. doi: 10.1084/jem.178.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porgador A, Gilboa E. J Exp Med. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celluzzi C M, Mayordomo J I, Storkus W J, Lotze M T, Falo L J. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zitvogel L, Mayordomo J I, Tjandrawan T, DeLeo A B, Clarke M R, Lotze M T, Storkus W J. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender A, Bui L K, Feldman M A, Larsson M, Bhardwaj N. J Exp Med. 1995;182:1663–1671. doi: 10.1084/jem.182.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohm W, Schirmbeck R, Elbe A, Melber K, Diminky D, Kraal G, van Rooijen N, Barenholz Y, Reimann J. J Immunol. 1995;155:3313–3321. [PubMed] [Google Scholar]
- 11.Bachmann M F, Lutz M B, Layton G T, Harris S J, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Eur J Immunol. 1996;26:2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 12.Norbury C C, Hewlett L J, Prescott A R, Shastri N, Watts C. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 13.Reis e Sousa C, Germain R N. J Exp Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacsovics B M, Rock K L. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 16.Schirmbeck R, Melber K, Reimann J. Eur J Immunol. 1995;25:1063–1070. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 17.Norbury C C, Chambers B J, Prescott A R, Ljunggren H G, Watts C. Eur J Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 18.Shen Z, Reznikoff G, Dranoff G, Rock K L. J Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- 19.Reis e Sousa C, Stahl P D, Austyn J M. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson R A, Watkins S C, Flynn J L. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Naito M, Steinman R M. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filgueira L, Nestle F O, Rittig M, Joller H I, Groscurth P. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 23.Svensson M, Stockinger B, Wick M J. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 24.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Ccastagnoli P. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 26.Oggioni M R, Pozzi G. Gene. 1996;169:85–90. doi: 10.1016/0378-1119(95)00775-x. [DOI] [PubMed] [Google Scholar]
- 27.Pozzi G, Oggioni M R, Medaglini D. In: Gram-Positive Bacteria As Vaccine Vehicles for Mucosal Immunization. Pozzi G, Wells J M, editors. Berlin: Springler; 1997. pp. 35–60. [Google Scholar]
- 28.Pozzi G, Oggioni M R, Manganelli R, Medaglini D, Fischetti V A, Fenoglio D, Valle M T, Kunkl A, Manca F. Vaccine. 1994;12:1071–1078. doi: 10.1016/0264-410x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 29.Pozzi G, Musmanno R A, Lievens P M-J, Oggioni M R, Plevani P, Manganelli R. Res Microbiol. 1990;141:659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
- 30.Glauert A M. Practical Methods in Electron Microscopy. Amsterdam: North–Holland; 1975. pp. 33–40. [Google Scholar]
- 31.Karttunen J, Sanderson S, Shastri N. Proc Natl Acad Sci USA. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medaglini D, Pozzi G, King T P, Fischetti V A. Proc Natl Acad Sci USA. 1995;92:6868–6872. doi: 10.1073/pnas.92.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medaglini D, Rush C M, Sestini P, Pozzi G C. Vaccine. 1997;15:1157–1163. doi: 10.1016/s0264-410x(97)00026-1. [DOI] [PubMed] [Google Scholar]
- 34.Rittig M G, Filgueira L, Dechant C A, Ricciardi-Castagnoli P, Groscurth P, Burmester G R. J Cell Biochem. 1995;21A:30. [Google Scholar]
- 35.Rittig M, Krause A, Haupl T, Shaible U, Modolell M, Kramer M, Lutjen-Drecoll E, Simon M, Burmester G. Infect Immun. 1992;60:4205–4212. doi: 10.1128/iai.60.10.4205-4212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rittig M, Haupl T, Burmester G R. Int Arch Allergy Immunol. 1994;103:4–10. doi: 10.1159/000236598. [DOI] [PubMed] [Google Scholar]
- 37.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella M, Scheidegger D, Palmer L K, Lane P, Lanzavecchia A, Alber G. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 40.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 41.Ingulli E, Mondino A, Khorutz A, Jenkins A K. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutz M B, Rovere P, Kleijmeer M J, Rescigno M, Abmann C U, Oorschot V M J, Geuze H J, Trucy J, Demandolx D, Davoust J, Ricciardi-Castagnoli P. J Immunol. 1997;159:3707–3716. [PubMed] [Google Scholar]
- 43.Wick M J, Pfeifer J D. Eur J Immunol. 1996;26:2790–2799. doi: 10.1002/eji.1830261135. [DOI] [PubMed] [Google Scholar]