Abstract
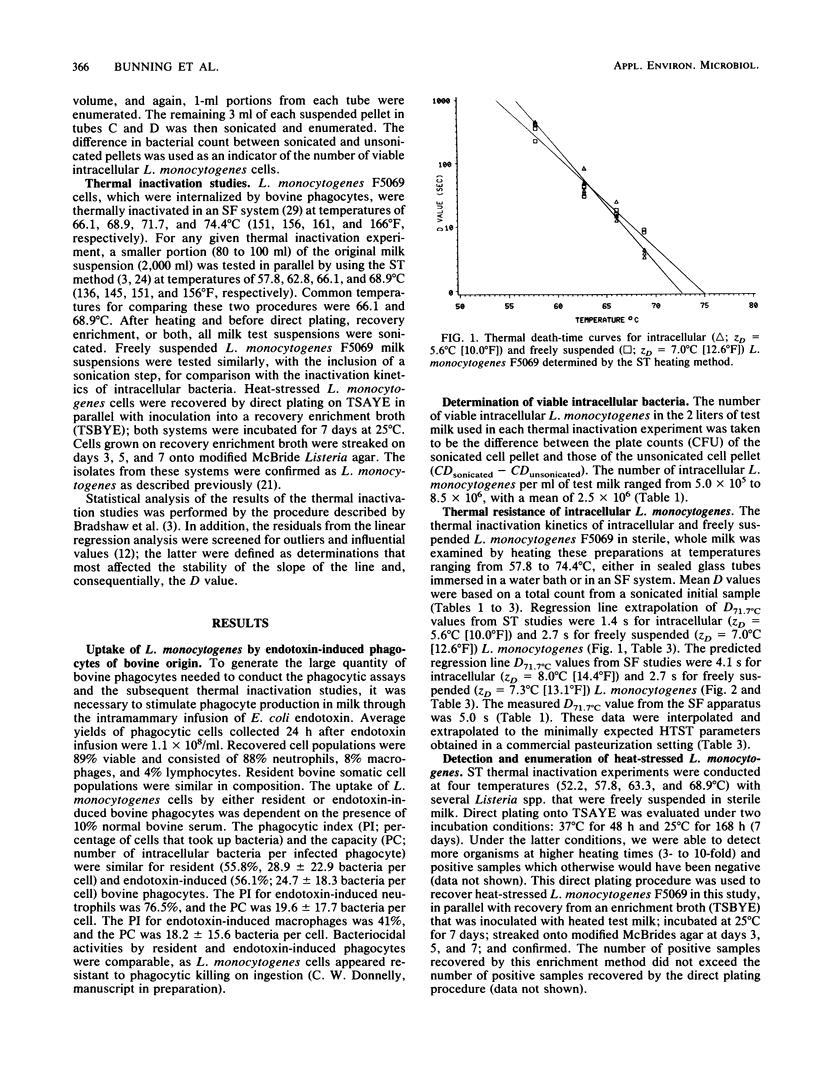
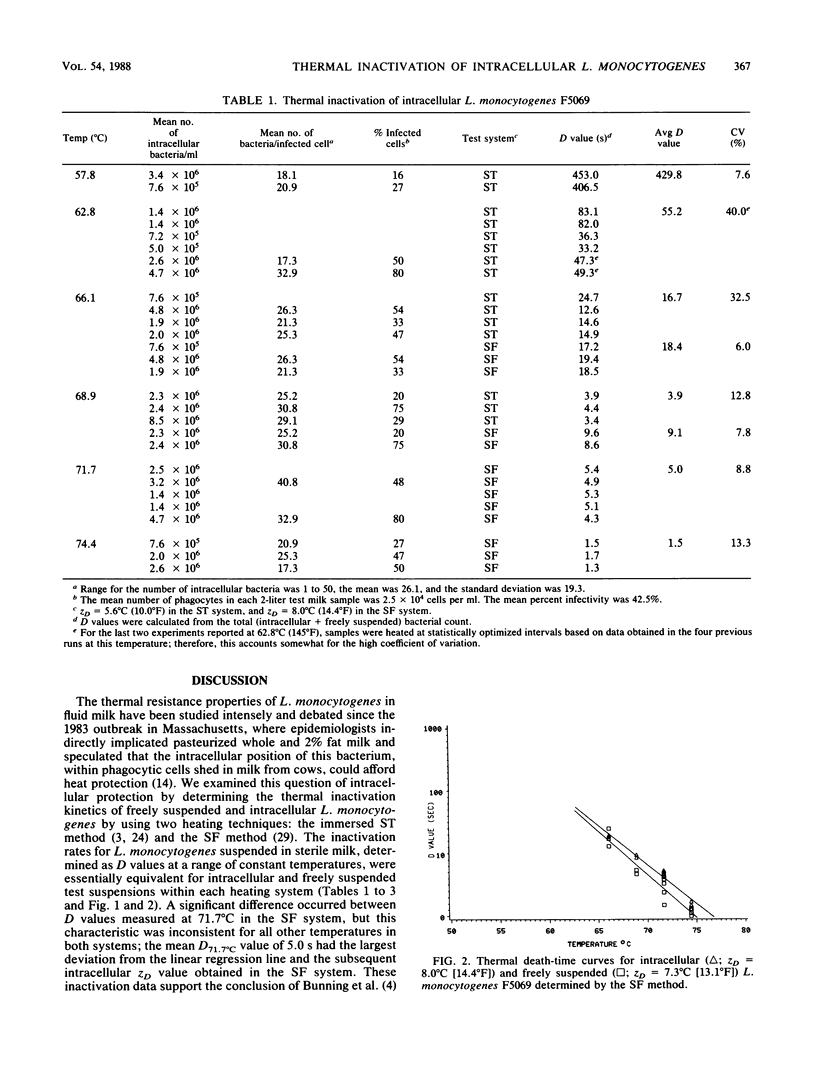
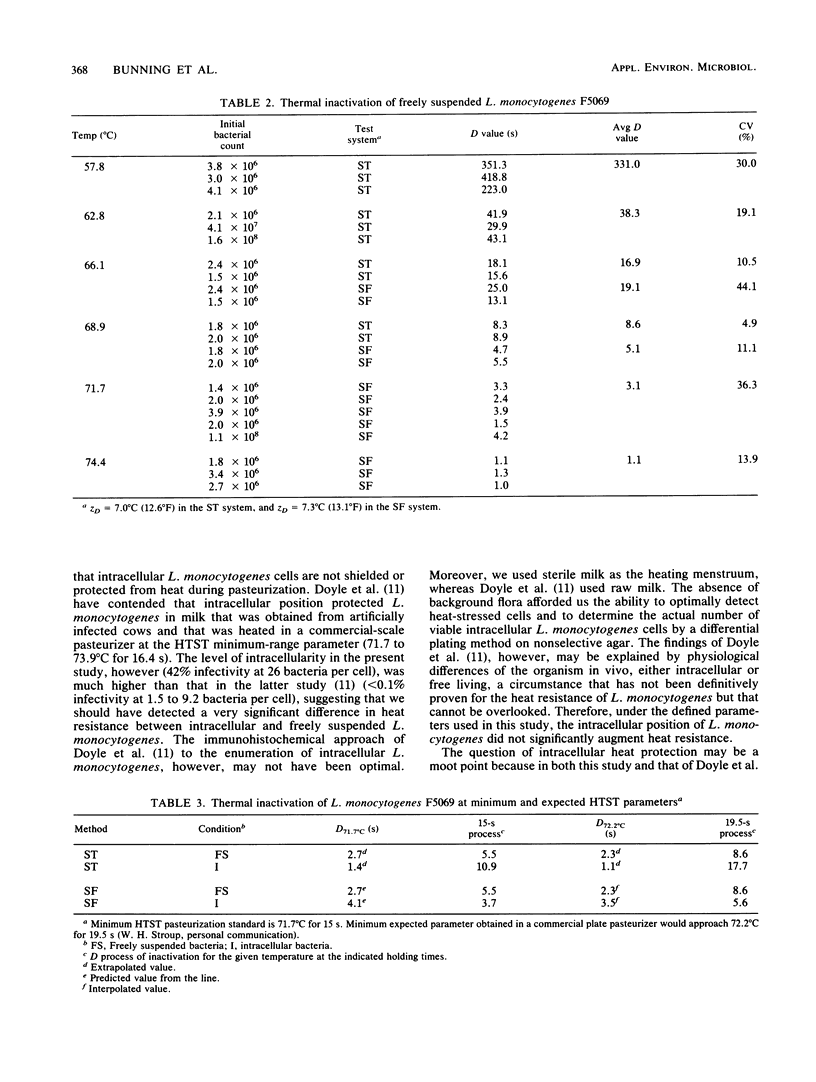
Thermal resistance of intracellular and freely suspended Listeria monocytogenes that was associated with a milkborne outbreak of listeriosis was studied by using the sealed tube and slug flow heat exchanger methods. Test temperatures for the former method were 57.8, 62.8, 66.1, and 68.9 degrees C (136, 145, 151, and 156 degrees F, respectively); whereas those for the latter method were 66.1, 68.9, 71.7, and 74.4 degrees C (151, 156, 161, and 166 degrees F, respectively). The heating menstruum was sterile, whole milk. The intracellular inoculum was generated from an in vitro phagocytosis reaction by using endotoxin-induced bovine milk phagocytes. The phagocyte population consisted of 88% neutrophils, 8% macrophages, and 4% lymphocytes. Neutrophils harbored the majority of intracellular L. monocytogenes. The mean level of infectivity in the phagocyte population was 43%, and there were 26.1 +/- 19.3 bacteria per cell (10(4) viable cells per ml of test milk). Initial bacterial counts for the freely suspended and intracellular experiments (the latter was based on a sonically disrupted sample) were 10(6) L. monocytogenes cells per ml. Heat-stressed bacteria were recovered by direct plating in parallel with recovery from an enrichment broth; both methods gave comparable results. The predicted D62.8 degrees C (145 degrees F) value for intracellular sealed tube studies was 53.8 s (ZD = 5.6 degrees C [10.0 degrees F]), indicating a safe 33.4 D margin of inactivation for vat pasteurization (62.8 degrees C for 30 min).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEARNS R. E., GIRARD K. F. The effect of pasteurization on Listeria monocytogenes. Can J Microbiol. 1958 Feb;4(1):55–61. doi: 10.1139/m58-007. [DOI] [PubMed] [Google Scholar]
- Beuchat L. R., Brackett R. E., Hao D. Y., Conner D. E. Growth and thermal inactivation of Listeria monocytogenes in cabbage and cabbage juice. Can J Microbiol. 1986 Oct;32(10):791–795. doi: 10.1139/m86-145. [DOI] [PubMed] [Google Scholar]
- Bunning V. K., Crawford R. G., Bradshaw J. G., Peeler J. T., Tierney J. T., Twedt R. M. Thermal resistance of intracellular Listeria monocytogenes cells suspended in raw bovine milk. Appl Environ Microbiol. 1986 Dec;52(6):1398–1402. doi: 10.1128/aem.52.6.1398-1402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuco A. V., Paape M. J., Nickerson S. C. In vitro study of polymorphonuclear leukocyte damage to mammary tissues of lactating cows. Am J Vet Res. 1986 Mar;47(3):663–668. [PubMed] [Google Scholar]
- Domínguez Rodriguez L., Fernández Garayzabal J. F., Vazquez Boland J. A., Rodriguez Ferri E., Suarez Fernández G. Isolation de micro-organismes du genre Listeria à partir de lait cru destiné à la consommation humaine. Can J Microbiol. 1985 Oct;31(10):938–941. [PubMed] [Google Scholar]
- Donnelly C. W., Baigent G. J. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl Environ Microbiol. 1986 Oct;52(4):689–695. doi: 10.1128/aem.52.4.689-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Glass K. A., Beery J. T., Garcia G. A., Pollard D. J., Schultz R. D. Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl Environ Microbiol. 1987 Jul;53(7):1433–1438. doi: 10.1128/aem.53.7.1433-1438.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Garayzábal J. F., Domínguez Rodríguez L., Vázquez Boland J. A., Blanco Cancelo J. L., Suárez Fernández G. Listeria monocytogenes dans le lait pasteurisé. Can J Microbiol. 1986 Feb;32(2):149–150. [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Lee W. H., McClain D. Improved Listeria monocytogenes selective agar. Appl Environ Microbiol. 1986 Nov;52(5):1215–1217. doi: 10.1128/aem.52.5.1215-1217.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTEL J. Die Morphologie, Kultur und Tierpathogenität des Corynebacterium infantisepticum. Zentralbl Bakteriol Orig. 1951 Jun 16;156(7):490–493. [PubMed] [Google Scholar]
- Ralovich B., Forray A., Mérö E., Málovics H., Százados I. New selective medium for isolation of L. monocytogenes. Zentralbl Bakteriol Orig. 1971;216(1):88–91. [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Stroup W. H., Dickerson R. W., Jr, Read R. B., Jr Two-phase slug flow heat exchanger for microbial thermal inactivation research. Appl Microbiol. 1969 Nov;18(5):889–892. doi: 10.1128/am.18.5.889-892.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J. T., Larkin E. P. Potential sources of error during virus thermal inactivation. Appl Environ Microbiol. 1978 Sep;36(3):432–437. doi: 10.1128/aem.36.3.432-437.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


