Abstract
Lactobacillus isolates able to colonize the surfaces of the nonsecreting epithelia in the stomachs of monoassociated ex-germfree mice were derived from Lactobacillus acidophilus 100-33. Strain 100-33 was originally isolated from pig feces and is unable to colonize the murine gastric epithelium. In experiments involving attempts genetically to transform the capacity to colonize the epithelium, cells of strain 100-33 were treated with muralytic enzymes and mixed with polyethylene glycol and genomic or plasmid DNA extracted from Lactobacillus fermentum RI. Strain RI was originally isolated from a conventional mouse and has the capacity to colonize the nonsecreting gastric epithelium. The mixtures containing cells, polyethylene glycol, and DNA were plated on a regeneration medium. After overnight incubation, the cells were washed from the plates and introduced by gastric gavage into germfree mice. Only mice that received regenerated 100-33 cells previously mixed with genomic DNA from strain RI had layers of gram-positive bacteria on the keratinized epithelia of their stomachs. Six isolates cultured from the washed gastric tissues of these animals were characterized. When a culture of each or a pool of cultures of the six were orally administered to germfree mice, layers of gram-positive bacterial cells were visible on the keratinized gastric epithelia of the animals within 1 to 3 weeks. Cells of all six, but not of strain 100-33, reacted with antibody made in rabbits to L. fermentum RI cells, as determined by an enzyme-linked immunosorbent assay. Nevertheless, all six had fermentation profiles identical to that of strain 100-33.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Rousseau M. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun. 1981 Dec;34(3):957–969. doi: 10.1128/iai.34.3.957-969.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser C. H., Stocker J. W., Gisler R. H. Methods for binding cells to plastic: application to solid phase immunoassays for cell-surface antigens. Methods Enzymol. 1981;73(Pt B):406–418. doi: 10.1016/0076-6879(81)73081-7. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Savage D. C. Models for study of the specificity by which indigenous lactobacilli adhere to murine gastric epithelia. Infect Immun. 1979 Dec;26(3):966–975. doi: 10.1128/iai.26.3.966-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
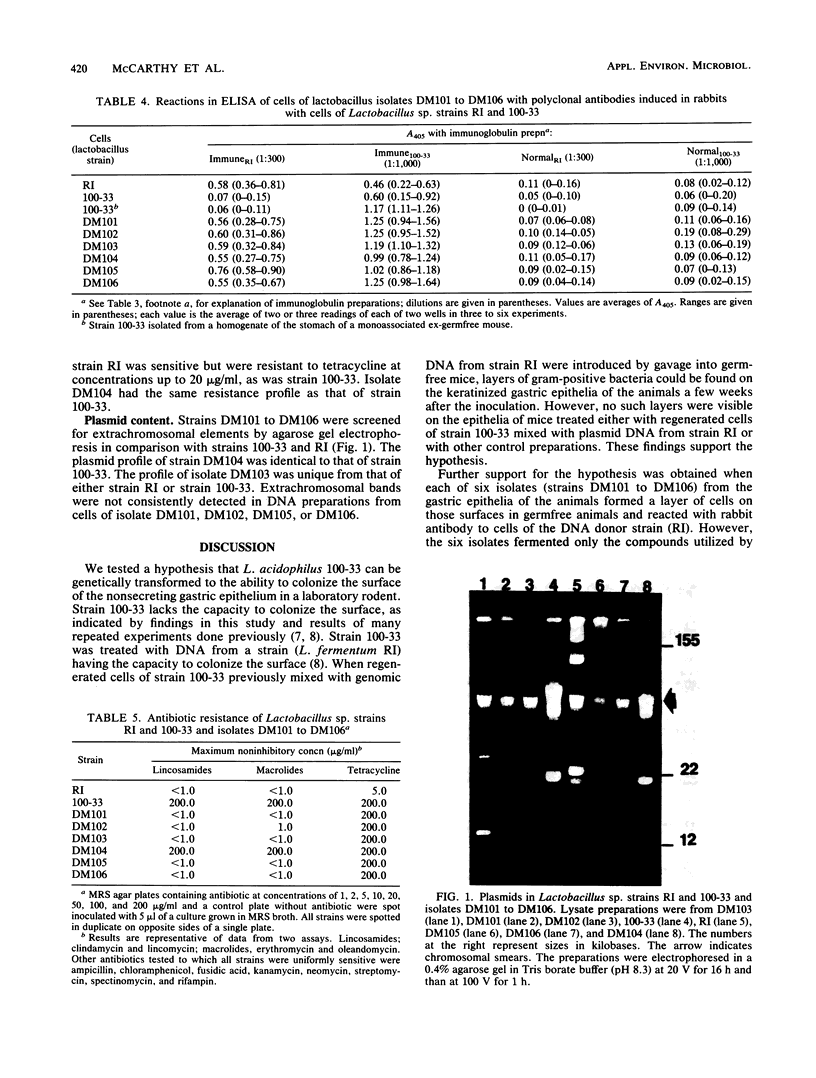
- Lin J. H., Savage D. C. Cryptic plasmids in Lactobacillus strains isolated from the murine gastrointestinal tract. Appl Environ Microbiol. 1985 Apr;49(4):1004–1006. doi: 10.1128/aem.49.4.1004-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Savage D. C. Genetic transformation of rifampicin resistance in Lactobacillus acidophilus. J Gen Microbiol. 1986 Aug;132(8):2107–2111. doi: 10.1099/00221287-132-8-2107. [DOI] [PubMed] [Google Scholar]
- Novick R., Sanchez-Rivas C., Gruss A., Edelman I. Involvement of the cell envelope in plasmid maintenance: plasmid curing during the regeneration of protoplasts. Plasmid. 1980 May;3(3):348–358. doi: 10.1016/0147-619x(80)90048-7. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Savage D. C. Lipoteichoic acids in Lactobacillus strains that colonize the mouse gastric epithelium. Appl Environ Microbiol. 1986 Aug;52(2):302–304. doi: 10.1128/aem.52.2.302-304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt D. D., Savage D. C. Kinetics of changes induced by indigenous microbiota in the activity levels of alkaline phosphatase and disaccharidases in small intestinal enterocytes in mice. Infect Immun. 1980 Jul;29(1):144–151. doi: 10.1128/iai.29.1.144-151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth R., An F. Y., Clewell D. B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986 Mar;165(3):831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]