Abstract
We used a catheter-based technique to achieve generalized cardiac gene transfer in vivo and to alter cardiac function by overexpressing phospholamban (PL) which regulates the activity of the sarcoplasmic reticulum Ca2+ ATPase (SERCA2a). By using this approach, rat hearts were transduced in vivo with 5 × 109 pfu of recombinant adenoviral vectors carrying cDNA for either PL, β-galactosidase (β-gal), or modified green fluorescent protein (EGFP). Western blot analysis of ventricles obtained from rats transduced by Ad.PL showed a 2.8-fold increase in PL compared with hearts transduced by Ad.βgal. Two days after infection, rat hearts transduced with Ad.PL had lower peak left ventricular pressure (58.3 ± 12.9 mmHg, n = 8) compared with uninfected hearts (92.5 ± 3.5 mmHg, n = 6) or hearts infected with Ad.βgal (92.6 ± 5.9 mmHg, n = 6). Both peak rate of pressure rise and pressure fall (+3, 210 ± 298 mmHg/s, −2, 117 ± 178 mmHg/s, n = 8) were decreased in hearts overexpressing PL compared with uninfected hearts (+5, 225 ± 136 mmHg/s, −3, 805 ± 97 mmHg/s, n = 6) or hearts infected with Ad.βgal (+5, 108 ± 167 mmHg/s, −3, 765 ± 121 mmHg/s, n = 6). The time constant of left ventricular relaxation increased significantly in hearts overexpressing PL (33.4 ± 3.2 ms, n = 8) compared with uninfected hearts (18.5 ± 1.0 ms, n = 6) or hearts infected with Ad.βgal (20.8 ± 2.1 ms, n = 6). These differences in ventricular function were maintained 7 days after infection. These studies open the prospect of using somatic gene transfer to modulate overall cardiac function in vivo for either experimental or therapeutic applications.
Keywords: sarcoplasmic reticulum, phospholamban, calcium, Ca2+ ATPase, adenovirus
The regulation of intracellular calcium is intimately related to the systolic and diastolic function of cardiac cells (1, 2). The sarcoplasmic reticulum (SR), which releases calcium during systole and takes it up during diastole, plays an integral part in controlling the synchronized movement of calcium in myocardial cells. The SR Ca2+ ATPase (SERCA2a) pump regulates the uptake of Ca2+ into the SR during diastole. The function of the SERCA2a pump is regulated in turn by phospholamban (PL) (3). In its unphosphorylated form, PL inhibits the SERCA2a pump whereas in its phosphorylated form, this inhibition is relieved. A decrease in SERCA2a activity has been identified in a number of animal models of heart failure and in human heart failure and an increase in the relative ratio of PL to SERCA2a appears to be an important characteristic of both experimental and human heart failure (3, 4). We have previously modeled such alteration in the PL/SERCA2a ratio by using adenoviral gene transfer to cardiocytes in vitro. Adenoviral overexpression of PL in vitro recapitulates many of the physiological abnormalities seen in heart failure, including prolonged relaxation and decreased contractile function. In contrast, overexpression of SERCA2a enhances relaxation and contractility of normal cardiomyocytes and rescues myocytes overexpressing PL from their abnormal phenotype (5, 6). Cardiac gene transfer has been previously achieved predominantly by direct injection into the myocardium or perfusion of an isolated coronary segment (7, 8). Either approach results in focal overexpression of the transgene and is therefore unlikely to effectively modulate global cardiac function. In this study we used a catheter-based technique to achieve highly effective transgene expression in rat heart in vivo. In vivo overexpression of PL resulted in profound physiological alterations in cardiac function including a decrease in left ventricular systolic pressure and an increase in diastolic pressure and a prolonged isovolumic relaxation. As in single cells overexpressing PL, these effects mimic abnormalities seen in experimental and human heart failure. Our data suggest that global adenoviral gene transfer to rodent hearts in vivo may be a useful tool for studying the molecular mechanisms regulating cardiac function. Overexpression of PL in particular creates an acquired phenotype that recapitulates many abnormalities seen in human heart failure and may provide a useful model for testing therapeutic interventions.
Materials & Methods
Construction of E1-Deleted Recombinant Adenoviral Vectors.
Three first generation type 5 recombinant adenoviruses (Ad) were used in these studies: Ad.βgal, Ad.PL, and Ad.EGFP. Ad.βgal, which carries a nuclear localizing form of β-galactosidase (β-gal), utilizes the dl327 backbone and was kindly provided by David Dichek (Gladstone Institute for Cardiovascular Diseases, San Francisco, CA) (9). The construction of Ad.PL has been described in detail (6). Ad.EGFP was similarly constructed through homologous recombination in 293 cells by using pJM17 as a source of adenoviral DNA. Ad.EGFP carries the cDNA for modified green fluorescence protein, EGFP, purchased from Clontech. In each, the exogenous cDNA has been substituted for E1 through homologous recombination in 293 cells and each contains a small deletion in E3. The recombinant viruses were prepared as high titer stocks by propagation in 293 cells as described (5, 6). The titer of stocks used for these studies measured by plaque assays were as follows: 3.1 × 1010 pfu/ml for Ad.PL, 2.7 × 1010 pfu/ml for Ad.βgal, and 2 × 1010 pfu/ml for Ad.EGFP with a particle/pfu ratio of 40:1, 37:1, and 50:1, respectively (viral particles/ml determined by using the relationship one absorbance unit at 260 nm is equal to 1012 viral particles/ml).
Adenoviral Delivery Protocol.
Rats were anesthetized with pentobarbital i.p. and placed on a ventilator. The chest was entered from the left side through the third intercostal space. The pericardium was opened and a 7-0 suture placed at the apex of the left ventricle. The aorta and pulmonary artery were identified. A 22 G catheter containing 200 μl of adenovirus was advanced from the apex of the left ventricle to the aortic root. The aorta and pulmonary arteries were clamped distal to the site of the catheter and the solution injected. The clamp was maintained for 10 s when the heart pumped against a closed system (isovolumically). This procedure allows the solution that contains the adenovirus to circulate down the coronary arteries and perfuse the heart without direct manipulation of the coronaries. After 10 s, the clamp on the aorta and pulmonary artery was released. After removal of air and blood, the chest was closed, and animals were extubated and transferred back to their cages.
Pressure Measurements.
Rats in the different treatment groups and at different stages following adenoviral gene transfer were anesthetized with 60 mg/kg of pentobarbital and mechanically ventilated. The chest was then opened through a mid-line incision and the heart exposed. A small incision was then made in the apex of the left ventricle and a 2.0 French high fidelity pressure transducer (Millar Instruments, Houston, TX) introduced into the left ventricle. Pressure measurements were digitized at 1 KHz and stored for further analysis. Left ventricular systolic pressure (LVSP), end-diastolic left ventricular pressure (LVDP), the maximal rates of pressure rise (+dP/dt) and of pressure fall (−dP/dt), and the time constant of relaxation (τ) were measured or derived in the different groups. The time course of isovolumic relaxation was measured by using the equation: P = Poe−t/τ + PB, where P is the left ventricular isovolumic pressure, Po is pressure at the time of peak −dP/dt, and PB is residual pressure.
Left Ventricular Dimension Measurements.
Multiple 1.3-mm piezoelectric crystals (Sonometrics, Alberta, Canada) were placed over the surface of the left ventricle along the short axis of the ventricle at the level of the mitral valve. The intercrystal distance was recorded along with the left ventricular pressure. Left ventricular pressure dimension loops were generated under different loading conditions by clamping the inferior vena cava. The end-systolic pressure–dimension relationship was obtained by producing a series of pressure dimension loops over a range of loading conditions and connecting the upper left hand corners of the individual pressure dimension loops to generate the maximal slope (Emax).
Preparation of SR Membranes from Left Ventricles.
To isolate SR membrane from hearts, we used a procedure modified from Harigaya and Schwartz (10). Briefly, left ventricular myocardium was suspended in a buffer containing 300 mmol/liter sucrose, 1 mmol/liter phenylmethylsulfonyl fluoride,and 20 mmol/liter Pipes (pH 7.4). The tissue was then disrupted with a homogenizer. The homogenates were centrifuged at 500 × g for 20 min. The resultant supernatant was centrifuged at 25,000 × g for 60 min to pellet the SR-enriched membrane. The pellet was resuspended in a buffer containing 600 mmol/liter KCl, 30 mmol/liter sucrose, and 20 mmol/liter Pipes, frozen in liquid nitrogen and stored at −70°C. Protein concentration was determined in these preparations by a modified Bradford procedure (11) with BSA for the standard curve (Bio-Rad).
Histochemistry.
Hearts were examined by immunohistochemistry to evaluate the expression of β-galactosidase. Hearts were fixed with PBS containing 0.5% glutarldehyde for 30 min and then in PBS with 30% sucrose for 30 min. The hearts were then permeabilized by incubation in solution containing sodium deoxycholate (0.01%) and Nonidet P-40 for 15 min. Then the hearts were incubated overnight in a solution containing 5-bromo-4-chloro3-indolyl β-d-galactopyranoside (X-Gal), and 10-μm sections were then cut and examined under light microscopy. Lungs, livers, and full-length aorta were also fixed and examined in a similar fashion.
Western Blot Analysis of PL and SERCA2a in SR Preparations.
SDS/PAGE was performed on the isolated membranes from cell cultures under reducing conditions on a 7.5% separation gel with a 4% stacking gel in a Miniprotean II cell (Bio-Rad). Proteins were then transferred to a Hybond-ECL nitrocellulose for 2 h. The blots were blocked in 5% nonfat milk in TRIS-buffered saline for 3 h at room temperature. For immunoreaction, the blot was incubated with 1:2,500 diluted mAbs to either SERCA2a, Na/Ca exchanger, ryanodine receptors, and calsequestrin (Affinity BioReagents, Golden, CO) or 1:2,500 diluted anti-cardiac PL monoclonal IgG (Upstate Biotechnology, Lake Placid, NY) for 90 min at room temperature. After washing, the blots were incubated in a solution containing peroxidase-labeled goat anti-mouse IgG (dilution 1:1000) for 90 min at room temperature. The blot was then incubated in a chemiluminescence system and exposed to an X-Omat x-ray film (Fuji Films) for 1 min. The densities of the bands were evaluated by using NIH image. Normalization was performed by dividing densitometric units of each membrane preparation by the protein amounts in each of these preparations. Serial dilution of the membrane preparations revealed a linear relationship between amounts of protein and the densities of the PL immunoreactive bands (data not shown).
RESULTS
Cardiac Gene Transfer.
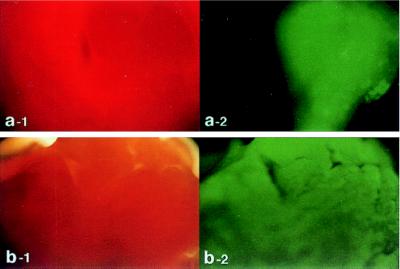
In vivo cardiac gene transfer was achieved by using a catheter inserted at the left ventricular apex and terminating just above the aortic valve. The adenoviral preparation was injected as the aorta and pulmonary artery were cross-clamped distal to the catheter tip for 10 s. During this period, the right and left ventricles became visibly pale as clear viral solution perfused the myocardium through the coronary arteries. During the procedure, heart rate decreased from ≈300 beat per minute (bpm) to ≈50 bpm, but recovered to baseline within 30 s of clamp release. Left ventricular systolic pressure increased to ≈300 mmHg and diastolic pressure to ≈25 mmHg. Ventricular pressure returned to baseline within 60 s of releasing the clamp. To examine the distribution of transgene expression, we used two adenoviruses carrying the reporter genes β-gal and EGFP, Ad.βgal and Ad.EGFP, respectively. As shown in Fig. 1, 2 days following delivery of Ad.EGFP with the catheter-based technique, the expression pattern observed was grossly homogeneous. In contrast, when Ad.EGFP was directly injected into the left ventricular wall the expression pattern was localized, whereas the surrounding tissue exhibited no background fluorescence.
Figure 1.
Rat hearts were transduced with Ad.EGFP by using either the catheter-based technique (b) or direct injection into the left ventricular wall (a). Forty-eight hours following delivery of adenovirus encoding for EGFP, the left ventricles of the hearts were removed and visualized with white light (a-1 and b-1) and at 510 nm with single excitation peak at 490 nm of blue light (a-2 and b-2). As shown in b-2, the expression pattern observed after catheter delivery is grossly homogeneous. In contrast, the expression pattern is localized after direct injection as shown in a-2. Of note, with the direct injection, the surrounding tissue exhibits no background fluorescence (a-2).
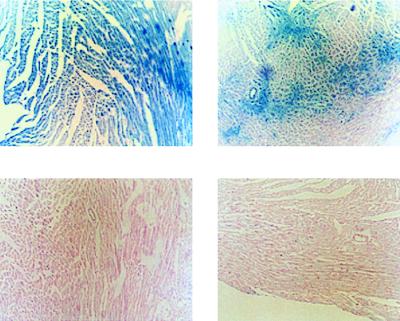
Hearts were also examined with immunohistochemistry to evaluate the microscopic distribution of transgene expression in vivo. As shown in Fig. 2, histochemical staining of ventricular cross-sections from hearts infected with Ad.βgal (day 2) revealed β-gal activity in myocytes not observed in Ad.PL-infected hearts. The distribution of β-gal was not uniform in all cross-sections. Certain areas had diffuse staining, whereas other sections had a more patchy distribution of expression. It is important to note that we used an adenovirus carrying a nuclear localized form of β-gala. Cytoplasmic β-gal activity is evident only in myocytes expressing the highest level of β-gal activity. Therefore, a ventricular section that typically reveals a small minority of muscle nuclei may underestimate the uniformity and the level of nuclear β-gal expression. To evaluate whether other tissues are infected, we histologically examined sections of aorta, liver, and lung following infection with Ad.βgal. There were no histological evidence of β-gal activity in the aorta; however, β-gal activity was present in the liver and lungs (data not shown). Infected rat hearts demonstrated an inflammatory response (7 days > 2 days postinfection); however, there was no evidence of disruption of normal myocardial architecture or collagen deposition.
Figure 2.
Expression of β-gal in left ventricular sections 2 days following infection with Ad.βgal and Ad.PL. (Upper) Photomicrographs of two left ventricular sections stained for β-gal 2 days following infection with Ad.βgal. These sections show the variability of β-gal expression within the same heart with the catheter-based method of gene delivery. (Lower) Photomicrographs of two left ventricular sections stained for β-gal 2 days following infection with Ad.PL. No β-gal expression is observed.
PL Overexpression.
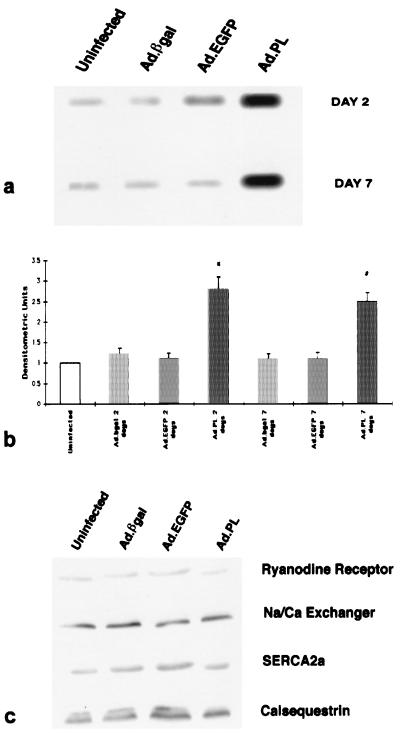
By using the technique described above, we transduced rat hearts with an adenovirus carrying PL (Ad.PL). As shown in Fig. 3a, infection with Ad.βgal or EGFP did not significantly alter the amount of PL in the rat hearts, whereas infection with Ad.PL increased the amount of PL by ≈2.5- to 3-fold. The increase in PL was also sustained at day 7. Quantification by densitometry of the immunoblots, depicted in Fig. 3b, shows an increase in PL at 2 days and at 7 days when compared with uninfected hearts. To verify that overexpression of PL in vivo does not affect other proteins involved in maintaining intracellular calcium homeostasis, Western blot analysis was performed for SERCA2a, the ryanodine receptor, calsequestrin, and the Na/Ca exchanger. As shown in Fig. 3c, infection with Ad.βgal, Ad.EGFP, or Ad.PL did not affect the level of these proteins.
Figure 3.
(a) Immunoblots of PL from crude membranes of left ventricles from control uninfected rats or rats 2 and 7 days after infection with 5 × 109 pfu of either Ad.βgal, Ad.EGFP, or Ad.RSV.PL. (b) Protein levels of PL in preparations from uninfected hearts (n = 8), preparations of hearts infected with Ad.βgal at day 2 (n = 6), preparations of hearts infected with Ad.EGFP at day 2 (n = 4), preparations of hearts infected with Ad.PL at day 2 (n = 8), preparations of hearts infected with Ad.βgal at day 7 (n = 6), preparations of hearts infected with Ad.EGFP at day 7 (n = 4), and preparations of hearts infected with Ad.PL at day 7 (n = 8). ∗, P < 0.05 compared with uninfected, Ad.βgal at 2 days, and Ad.EGFP at 2 days. #, P < 0.05 compared with Ad.βgal at 2 days, and Ad.EGFP at 7 days. There were no significant differences between the PL protein levels in the uninfected group and Ad.βgal or Ad.EGFP (P > 0.2). (c) Immunoblots with mAbs to the of ryanodine receptor, Na/Ca exchanger, SERCA2a, and calsequestrin from crude membrane of left ventricles after 2 days of infection with 5 × 109 pfu of either Ad.βgal, Ad.EGFP, or Ad.RSV.PL.
Effects of PL Overexpression on Hemodynamic Measurements.
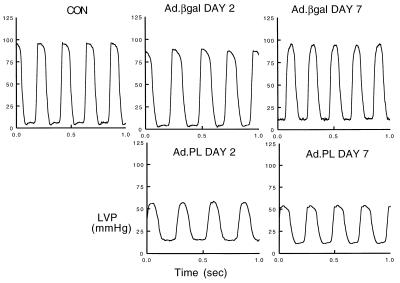
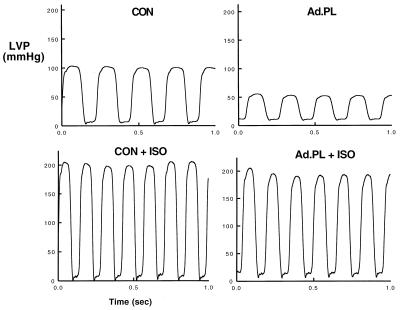
We next examined the physiological consequences of adenoviral gene transfer of β-gal or PL to rat hearts in vivo. As shown in Table 1, β-gal infection did not alter heart rate or any of the hemodynamic parameters examined compared with uninfected hearts at day 2 or day 7. Infection with Ad.PL did not significantly change heart rate at 2 days; however, at 7 days following infection with Ad.PL there was a trend toward an increase in heart rate. As shown in Fig. 4 and Table 1, the LVSP was significantly decreased in hearts overexpressing PL at both 2 and 7 days following infection, whereas left ventricular diastolic pressure (LVDP) was significantly increased in these hearts when compared with uninfected controls and to hearts infected with Ad.βgal. The peak rate of pressure rise (+dP/dt), a reflection of systolic function, was significantly decreased in hearts overexpressing PL at both 2 and 7 days. The peak rate of pressure fall, an index of diastolic function, was significantly decreased in hearts overexpressing PL compared with uninfected or Ad.βgal-infected hearts. The time constant of relaxation, which is an index of active relaxation, was significantly prolonged in hearts overexpressing PL compared with uninfected or Ad.βgal-infected hearts.
Table 1.
Systolic and diastolic parameters
| HR, min−1 | LVSP, mmHg | LVDP, mmHg | +dP/dt, mmHg/sec | −dP/dt, mmHg/sec | τ, msec | n | |
|---|---|---|---|---|---|---|---|
| Uninfected | 302 ± 21 | 92.5 ± 3.5 | 5.2 ± 1.1 | 5,225 ± 136 | −3,805 ± 97 | 18.5 ± 1.0 | 6 |
| Ad.βgal day 2 | 286 ± 19 | 92.6 ± 5.9 | 6.3 ± 1.6 | 5,108 ± 167 | −3,765 ± 121 | 20.8 ± 2.1 | 6 |
| Ad.PL day 2 | 270 ± 26 | 58.3 ± 12.9* | 12.7 ± 2.9* | 3,210 ± 298* | −2,117 ± 178* | 33.4 ± 3.2* | 8 |
| Ad.βgal day 7 | 290 ± 22 | 88.5 ± 5.0 | 6.5 ± 1.3 | 5,032 ± 234 | −3,668 ± 112 | 21.1 ± 2.4 | 6 |
| Ad.PL day 7 | 342 ± 31 | 54.2 ± 8.2† | 9.8 ± 2.3 | 3,345 ± 311† | −2,345 ± 154† | 32.4 ± 1.9† | 8 |
All data presented as mean ± SD. LVSP, left ventricular systolic pressure; LVDP, left ventricular end-diastolic pressure; HR, heart rate; +dP/dt, maximal rate of pressure rise; −dP/dt, maximal rate of pressure fall; τ, time constant of relaxation; n, number of hearts.
P < 0.01 compared to uninfected and Ad.βgal, day 2.
P < 0.01 compared to Ad.βgal, day 7.
Figure 4.
Left ventricular pressure measurements from rats that were either uninfected (CON) or infected with Ad.βgal or Ad.PL 2 and 7 days after infection as indicated. Hearts infected with Ad.PL had a decrease in systolic pressure, elevation of diastolic pressure, and prolongation of the relaxation phase.
Effect of Isoproterenol.
To verify the specificity of the profound hemodynamic effects observed after PL overexpression, we pharmacologically inhibited PL with isoproterenol, inducing the phosphorylation of PL thereby removing its inhibition of SERCA2a. The ventricular function after isoproterenol reflects the intrinsic SERCA2a activity. Therefore, induction of PL phosphorylation by isoproterenol should result in the same hemodynamic profile in both Ad.PL and uninfected hearts, reflecting their similar and unaffected intrinsic SR ATPase activity. As seen in Fig. 5, isoproterenol increased LVSP and relaxation in hearts overexpressing PL (203 ± 14 mmHg) to the same level as in uninfected (212 ± 12 mmHg) hearts. In addition, the isovolumic relaxation parameter τ in hearts infected with Ad.PL (12.8 ± 1.2 ms, n = 6) was decreased to levels similar to uninfected hearts (13.0 ± 1.1 ms, n = 6).
Figure 5.
Left ventricular pressure measurements during infusion of 0.1 μg/kg/min of isoproterenol in an uninfected rat heart and rat hearts infected with Ad.PL (5 × 109 pfu, day 2).
Pressure–Dimension Relationship.
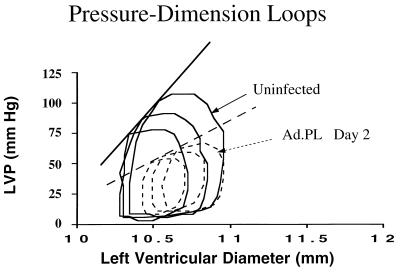
To further define ventricular function, pressure–dimension analysis was performed in a subset of animals. The relationship between developed pressure and ventricular dimension under different loading conditions allows accurate assessment of systolic and diastolic function. Fig. 6 shows a pressure–dimension relationship in an uninfected heart and a heart infected with Ad.PL at 2 days. The pressure–dimension loop is shifted to higher ventricular dimensions in the heart overexpressing PL. To alter loading conditions, we clamped the inferior vena cava in the open-chested animals, thereby reducing ventricular volume. This procedure enabled us to calculate the maximal end-systolic pressure–dimension relationship with a series of measurements made under varying preload conditions. The maximal slope of the end-systolic pressure–dimension relationship was lower in hearts overexpressing PL, indicating a diminished state of intrinsic myocardial contractility: 72 ± 21 mmHg/mm vs. 128 ± 24 mmHg/mm in Ad.PL-infected hearts (n = 6) compared with uninfected hearts (n = 6) [P < 0.05].
Figure 6.
Left ventricular pressure vs. left ventricular dimension detected by piezoelectric crystals in a control uninfected heart and in a heart infected with Ad.PL (5 × 109 pfu, day 2) under different loads obtained by clamping the inferior vena cava.
DISCUSSION
In this study we used a catheter-based technique to achieve global cardiac transduction with recombinant adenoviral vectors. Transgene expression was diffuse and relatively homogeneous throughout the myocardium. Importantly, transduction with adenoviral vectors carrying reporter genes did not significantly alter any of the physiological parameters. In contrast, cardiac overexpression of PL at 2- to 3-fold the endogenous level dramatically modulated global left ventricular function, recapitulating many of the abnormalities of human and experimental heart failure.
Cardiac Gene Transfer.
Although prior studies have demonstrated the feasibility of cardiac gene transfer, the general utility of these approaches has been limited by either very focal expression after in vivo transduction or the requirement of ex vivo infection for more diffuse transduction (12–15). Intracoronary catheter delivery of an adenovirus encoding β-gal achieved transduction of about 30% of the myocytes in the distribution of the coronary artery (12). Direct injection of adenovirus into the ventricular walls also induced significant expression of reporter constructs; however, the expression was focal and the injections within the myocardium cause needle damage (7, 8). More recently, Donahue et al. (15) reported effective gene transfer to the heart by using intracoronary perfusion in explanted hearts at physiological temperature. This group identified a number of parameters that influence the efficiency of adenoviral gene transfer. These included (i) the use of crystalloid solution as opposed to whole blood, (ii) coronary flow rate, (iii) exposure time, (iv) virus concentration, and (v) temperature. The success of the approach described here may reflect optimization of these parameters. The amount of virus used was 5 × 109 pfu diluted in 200 μl of solution and 150 μl from the left ventricular blood volume, resulting in a final concentration ≈1.5 × 1010 pfu/ml. The concentration of the adenovirus was not diluted during the cross-clamping because blood return to the left atrium was minimized by the simultaneous clamping of the pulmonary artery. This concentration of virus is high compared with previous studies (7, 8, 15). In addition, the blood was diluted thereby decreasing the inhibitory effects of red blood cells on the efficiency of gene delivery. Even though we did not directly measure flow rates, cross-clamping increases perfusion pressure down the coronaries, allowing maximal opening of capillaries and optimizing the myocardial area of virus exposure. Furthermore, during cross-clamping the heart rate was decreased, thereby increasing diastolic time. During diastole, the left ventricular end-diastolic pressure was not significantly increased because blood return to the left ventricle was blocked by clamping of the pulmonary artery. Therefore perfusion of the virus could occur at relatively low downstream pressure and the endocardium could be efficiently infected. Because attachment of the adenovirus to cells is temperature dependent, delivering the virus in vivo allowed us to use the optimal temperature for adenoviral gene transfer, 37°C. Donahue et al. (15) have also shown that in vitro exposure of 10 s at a concentration of 107 pfu/ml results in ≈60% transduction studies. In the present study, where the delivery conditions have been optimized, the exposure time was 10 s at a much higher concentration of ≈1010 pfu/ml. In summary, many of the critical parameters previously characterized as important for effective gene transfer to myocytes, in vitro or ex vivo, are optimized during the described approach to cardiac gene transfer in vivo. Undoubtedly this contributes to its success and raises the possibility that in vivo global cardiac gene transfer will be feasible in other experimental or even clinical settings.
To assess the degree of gene expression with the delivery method described, we used two approaches. First, we visualized the whole heart by fluorescent microscopy after injection of Ad.EGFP. Second, we used histochemical staining to assess the microscopic distribution of transgene expression. Ad.EGFP infection produced grossly homogeneous expression. Of note, injection of the hearts with other viruses (carrying genes other than EGFP) exhibited no background fluorescence in this emission range, and direct injection into the ventricular wall of Ad.EGFP demonstrated intense green fluorescence at the site of injection but no background fluorescence in the surrounding tissue (Fig. 1). To further evaluate the microscopic distribution of transgene expression in vivo, the transduced hearts were examined with immunohistochemistry. We found that the distribution of β-gal staining was not uniformly diffuse in all cross-sections. Certain areas had diffuse staining, whereas in other sections a more patchy distribution of expression was observed. This staining may underestimate the extent of transgene expression both because the nuclear-localized β-gal construct used exhibits cytoplasmic activity only in highly expressing cells and because histochemical staining of β-gal is relatively insensitive. Lower levels of expression may not be detected by this method but may be sufficient to induce physiological changes, depending on the transgene expressed. Together our data with reporter constructs demonstrate highly effective gene transfer to adult rat hearts in vivo in a relatively homogeneous distribution.
Even though our delivery method was specifically targeted to the heart, we found expression of the reporter transgene in other tissues in the body, such as lung and liver but not aorta, by using histochemical staining. Other investigators have found extracardiac transgene expression following in vivo injection of adenovirus into the heart when using a nonspecific promoter (16, 17). The use of tissue-specific promoters may obviate this problem in the future.
PL Overexpression.
Although such effective cardiac gene transfer with reporter constructs is encouraging, ultimately the utility of this approach depends on the ability to achieve functionally meaningful expression of biologically relevant transgenes. As a rigorous test of the system we chose to overexpress PL, an integral component of the SR thought to act only on the cell in which it is expressed. Expression of a secretory protein or a molecule with a significant bystander effect would be predicted to require less effective transduction to achieve a biological effect. Despite the stringent requirements of cell autonomous activity, PL had a dramatic effect on global left ventricular function.
PL is an integral protein of the SR of mammalian myocardium and regulates the Ca2+ ATPase that transports Ca2+ into the SR (3, 4). In the unphosphorylated state, PL inhibits the SERCA2a by reducing its affinity for Ca2+. Phosphorylation of PL at either the Ser-16 site by cAMP-dependent protein kinase or the Thr-17 site by calmodulin-dependent mechanisms removes the inhibition to the SERCA2a. PL has been shown to be phosphorylated in situ and to contribute significantly to the positive inotropic response and the relaxant effects of β-agonism in the working heart (18). The expression of PL relative to SERCA2a has been shown to be altered in a number of diseased states (4, 19). Both human and experimental heart failure are associated with an increased ratio of PL/SERCA2a and are characterized by a prolonged calcium transient and impaired relaxation (20). In previous studies, we have shown that increasing levels of PL relative to SERCA2a in isolated cardiomyocytes prolonged the relaxation phase of the Ca2+-transient, decreased Ca2+ release, and increased resting Ca2+ (6). These abnormalities recapitulate in single cells many of the fundamental pathophysiologic abnormalities seen in heart failure. In the present study, overexpression of PL resulted in a decrease in systolic pressure, an increase in diastolic pressure, and a large increase in the time constant for isovolumic relaxation. Isovolumic relaxation, which is an index of active relaxation and reflects the removal of Ca2+ from the myofilaments into the SR, was significantly prolonged in the hearts overexpressing PL. This finding was also evident in the pressure–dimension relationship, which was characterized by a slowed early diastolic decline and opening of the mitral valve at higher filling pressures. The decrease in systolic pressure most likely reflects a decrease in SR Ca2+ loading. The SERCA2a is important both during relaxation by controlling the rate and amount of Ca2+ sequestered and during contraction by releasing the Ca2+ that is taken up by the SR. Overexpression of PL decreases SERCA2a activity, ultimately resulting in diminished systolic pressure and elevated diastolic pressure. In animals overexpressing PL, diastolic pressure was also elevated. PL has been shown to play a key role in modulating the response of agents that increase cAMP levels in cardiomyocytes (18, 21). Because cAMP-induced phosphorylation of PL reduces its inhibition of SERCA2a, we evaluated the effects of isoproterenol on the ventricular performance in hearts overexpressing PL. At maximal isoproterenol stimulation, the time course of isovolumic relaxation was decreased to levels similar to uninfected hearts and the LVSP was increased to levels similar to uninfected hearts. These results strongly suggest that the abnormalities observed in ventricular function in the hearts overexpressing PL are specifically the result of PL-mediated inhibition of SR ATPase. Pharmacological release of this inhibition restores ventricular function to the same level as uninfected hearts, reflecting the intrinsic SERCA2a activity that is the same in both cases.
A transgenic approach of overexpressing PL has been undertaken in mice. However, adenoviral transduction offers several advantages. In transgenic animals overexpressing PL, developmental adaptation to higher levels of PL occurs with up-regulation of other important excitation-contraction proteins such as the ryanodine receptor, thereby masking or diluting the effects of transgene overexpression (22). More recently, transgenic animals overexpressing another key protein involved in calcium regulation in myocytes, SERCA2a, have been shown to induce increases in the mRNA for PL and the sodium-calcium exchanger (23, 24). These compensatory alterations make it difficult to assess the specific effect of increasing PL on cardiac function. In our studies, overexpression of PL did not significantly alter protein expression of the ryanodine Ca2+-releasing channels, SERCA2a, Na/Ca exchanger, or calsequestrin, which are all involved in intracellular calcium handling. However, adenoviruses have significant disadvantages that include the transient nature of overexpression of the desired gene and the immune/inflammatory response they produce and which was also present in our infected hearts. These shortcomings of the first generation adenoviruses limit their use in animal models over prolonged periods of time.
Although we noted extracardiac transgene expression, it is unlikely to account for the phenotype we observed. Although PL may regulate the SR Ca2+ pump in aortic smooth muscle cells (25), we found no histochemical evidence of β-gal expression in aortae. There is no known functional roles for these pathways in liver or lung, where we did detect extracardiac transgene expression. Moreover, we examined indices of systolic and diastolic ventricular performance that are independent of load (or changes in aortic compliance) and reflect intrinsic cardiac function. Our physiological data demonstrate the feasibility of achieving important functional cardiac effects through in vivo somatic gene transfer of a cell autonomous protein. Moreover, the abnormalities developed after PL overexpression in vivo reflect the abnormalities seen in experimental and human heart failure. Therefore, somatic gene transfer with Ad.PL can create both in vitro (6) and in vivo models of heart failure that should facilitate studies of pathophysiology and investigation of potential therapeutic interventions.
Conclusion.
In conclusion, the present study demonstrates highly effective gene transfer to rat heart in vivo. By using this technique, we overexpressed PL, thereby recapitulating many of the abnormalities observed in heart failure. Together these studies open the prospect of using somatic gene transfer to modulate overall cardiac function in vivo for either experimental or therapeutic applications.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: HL 50361 and HL 57623 (R.J.H.); HL 54202, HL 59521, and AI 40970 (A.R.); and HL 49574 and HL 52249 (J.K.G.), and by donations to the Katherine Catani Memorial Fund (A.R.).
ABBREVIATIONS
- PL
phospholamban
- EGFP
modified green fluorescent protein
- SR
sarcoplasmic reticulum
- SERCA2a
SR Ca2+ ATPase
- Ad
adenovirus
- β-gal
β-galactosidase
- LVSP
left ventricular systolic pressure
References
- 1.Barry W H, Bridge J H B. Circulation. 1993;87:1806–1815. doi: 10.1161/01.cir.87.6.1806. [DOI] [PubMed] [Google Scholar]
- 2.Gwathmey J K, Bentivegna L A, Ransil B J, Grossman W, Morgan J P. Cardiovasc Res. 1993;27:199–203. doi: 10.1093/cvr/27.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Koss K L, Kranias E G. Circulation Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 4.Arai M, Matsui H, Periasamy M. Circ Res. 1994;74:555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar R J, Kang J X, Gwathmey J K, Rosenzweig A. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar R J, Schmidt U, Kang J X, Matsui T, Rosenzweig A. Circ Res. 1997;81:145–153. doi: 10.1161/01.res.81.2.145. [DOI] [PubMed] [Google Scholar]
- 7.Guzman R J, Lemarchand P, Crystal R G, Epstein S E, Finkel T. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 8.French B A, Mazur W, Geske R S, Bolli R. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 9.Dong G, Schulick A, DeYoung M B, Dichek D A. J Biol Chem. 1996;271:29969–29977. doi: 10.1074/jbc.271.47.29969. [DOI] [PubMed] [Google Scholar]
- 10.Harigaya S, Schwartz A. Circulation Res. 1969;25:781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- 11.Bradford M. Anal Biochem. 1976;72:248–260. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Barr E, Carroll J, Kalynych A M, Tripathy S K, Kozarsky K, Wilson J M, Leiden J M. Gene Ther. 1994;1:51–58. [PubMed] [Google Scholar]
- 13.Kirshenbaum L A, MacLennan W R, Mazur W, French B A, Schneider M D. J Clin Invest. 1993;92:381–387. doi: 10.1172/JCI116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kass-Eisler A, Falck-Pedersen E, Alvira M, Rivera J, Buttrick P M, Wittenberg B A, Cipriani L, Leinwand L A. Proc Natl Acad Sci USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donahue J K, Kikkawa K, Johns D C, Marban E, J. L. Proc Natl Acad Sci USA. 1997;94:4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kass-Eisler A, Falck-Pedersen E, Alvira M, Rivera J, Buttrick P M, Wittenberg B A, Cipriani L, Leinwand L A. Proc Natl Acad Sci USA. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 18.Luo W, Grupp I L, Harrer J, Ponniah S, Grupp G, Duffy J J, Doetschman T, Kranias E G. Circulation Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien P J, Gwathmey J K. Cardiovasc Res. 1995;30:394–404. doi: 10.1016/s0008-6363(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 20.Gwathmey J K, Morgan J P. Pflügers Arch. 1993;422:599–608. doi: 10.1007/BF00374008. [DOI] [PubMed] [Google Scholar]
- 21.Karczewski P, Bartel S, Krause E-G. Biochem J. 1990;266:115–122. doi: 10.1042/bj2660115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu G, Luo W, Slack J P, Tilgmann C, Sweet W E, et al. Circ Res. 1996;79:1064–1076. doi: 10.1161/01.res.79.6.1064. [DOI] [PubMed] [Google Scholar]
- 23.He H, Giordano F J, Hilal-Dandan R, Choi D J, Rockman H A, et al. J Clin Invest. 1997;100:380–389. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giordano F J, He H, McDonough P, Meyer M, Sayen M R, Dillmann W H. Circulation. 1997;96:400–403. doi: 10.1161/01.cir.96.2.400. [DOI] [PubMed] [Google Scholar]
- 25.Lalli J, Harrer J M, Luo W, Kranias E G, Paul R J. Circ Res. 1997;80:506–513. doi: 10.1161/01.res.80.4.506. [DOI] [PubMed] [Google Scholar]