Abstract
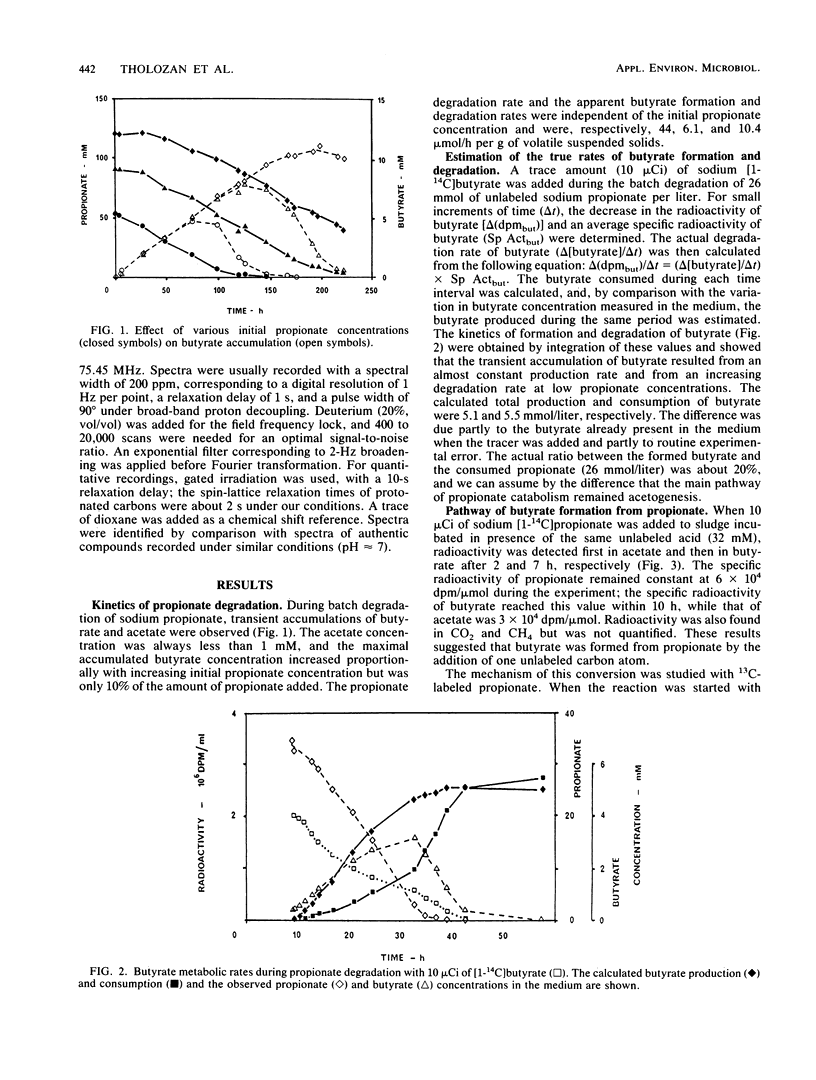
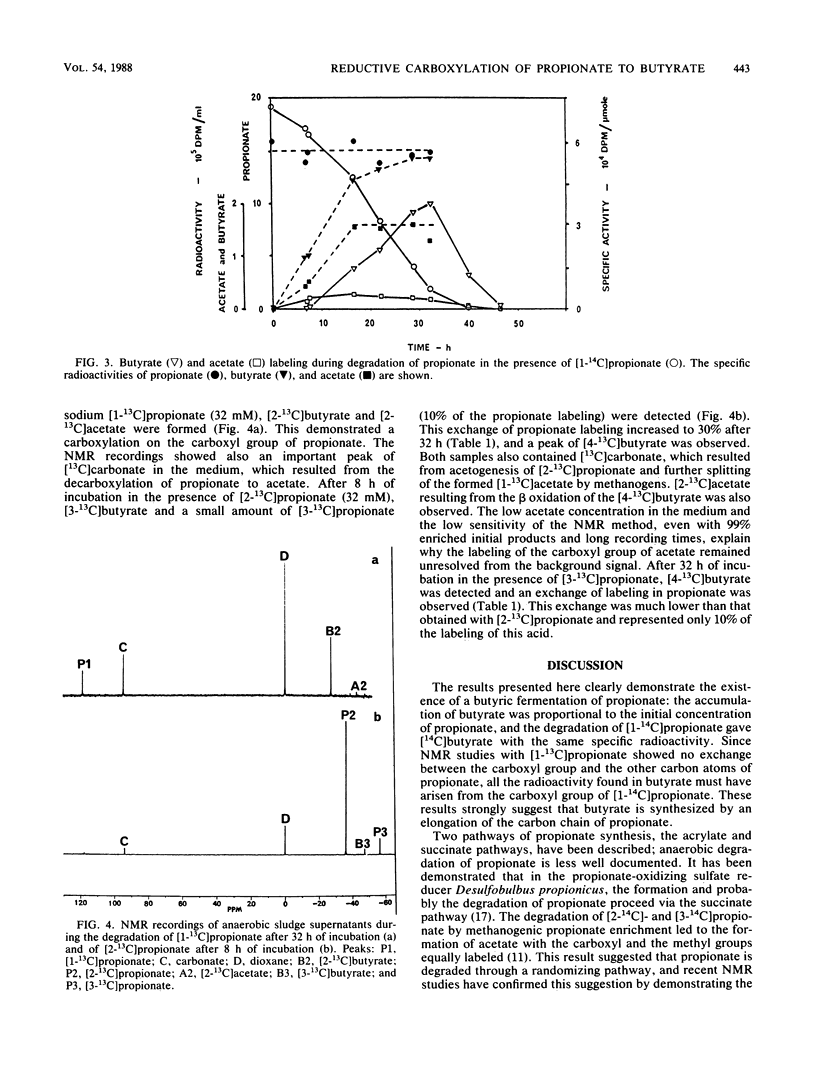
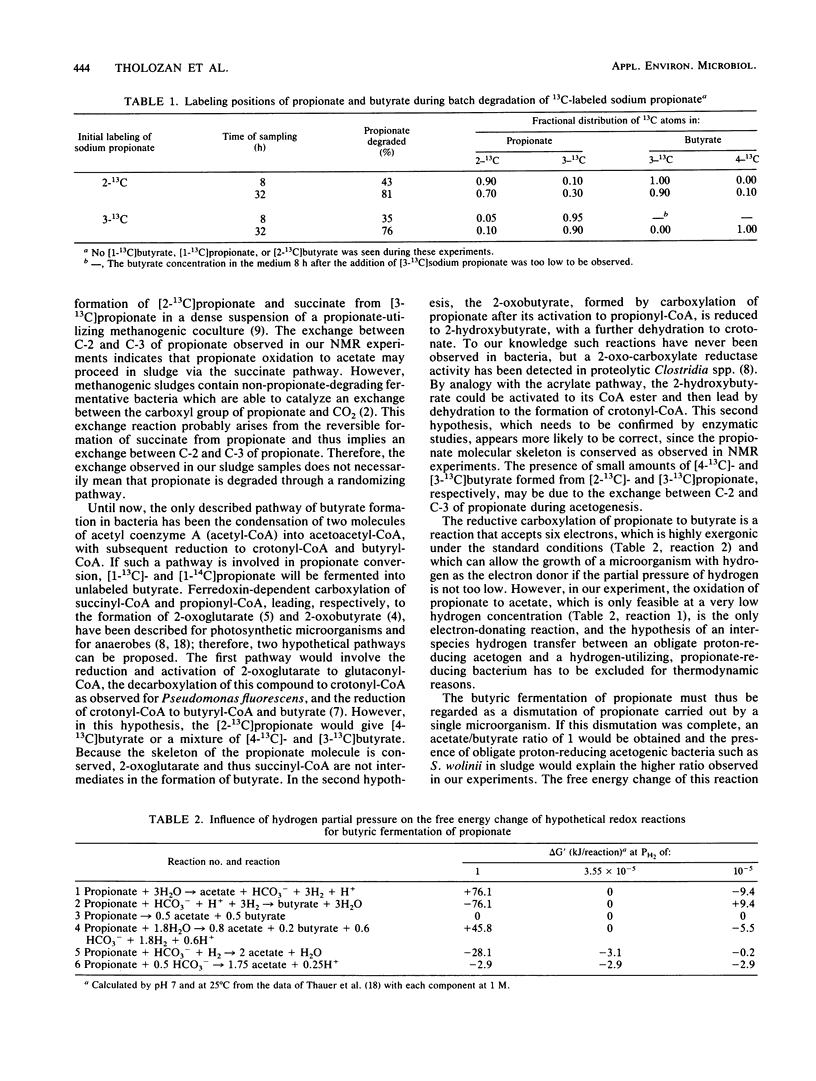
During the batch degradation of sodium propionate by the anaerobic sludge from an industrial digestor, we observed a significant amount of butyrate formation. Varying the initial propionate concentrations did not alter the ratio of maximal butyrate accumulation to initial propionate concentration within a large range. By measuring the decrease in the radioactivity of [1-14C]butyrate during propionate degradation, we estimated that about 20% of the propionate was converted to butyrate. Labeled butyrate was formed from [1-14C]propionate with the same specific radioactivity, suggesting a possible direct pathway from propionate to butyrate. We confirmed this hypothesis by nuclear magnetic resonance studies with [13C]propionate. The results showed that [1-13C]-, [2-13C]-, and [3-13C]propionate were converted to [2-13C]-, [3-13C]-, and [4-13C]butyrate, respectively, demonstrating the direct carboxylation on the carboxyl group of propionate without randomization of the other two carbons. In addition, we observed an exchange reaction between C-2 and C-3 of the propionate, indicating that acetogensis may proceed through a randomizing pathway. The physiological significance and importance of various metabolic pathways involved in propionate degradation are discussed, and an unusual pathway of butyrate synthesis is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R. Propionate exchange reactions in methanogenic ecosystems. Appl Environ Microbiol. 1984 Oct;48(4):863–864. doi: 10.1128/aem.48.4.863-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C. The synthesis of alpha-ketoglutarate from succinate and carbon dioxide by a subcellular preparation of a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1212–1218. doi: 10.1073/pnas.54.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B. Role of ferredoxin in the synthesis of alpha-ketobutyrate from propionyl coenzyme A and carbon dioxide by enzymes from photosynthetic and nonphotosynthetic bacteria. J Biol Chem. 1969 Aug 10;244(15):4218–4223. [PubMed] [Google Scholar]
- Giesel H., Simon H. On the occurrence of enoate reductase and 2-oxo-carboxylate reductase in clostridia and some observations on the amino acid fermentation by Peptostreptococcus anaerobius. Arch Microbiol. 1983 Aug;135(1):51–57. doi: 10.1007/BF00419482. [DOI] [PubMed] [Google Scholar]
- Koch M., Dolfing J., Wuhrmann K., Zehnder A. J. Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol. 1983 Apr;45(4):1411–1414. doi: 10.1128/aem.45.4.1411-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


