Abstract
Thermoanaerobacter ethanolicus (ATCC 31550) has primary and secondary alcohol dehydrogenases. The two enzymes were purified to homogeneity as judged from sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration. The apparent Mrs of the primary and secondary alcohol dehydrogenases are 184,000 and 172,000, respectively. Both enzymes have high thermostability. They are tetrameric with apparently identical subunits and contain from 3.2 to 5.5 atoms of Zn per subunit. The two dehydrogenases are NADP dependent and reversibly convert ethanol and 1-propanol to the respective aldehydes. The Vm values with ethanol as a substrate are 45.6 μmol/min per mg for the primary alcohol dehydrogenase and 13 μmol/min per mg for the secondary alcohol dehydrogenase at pH 8.9 and 60°C. The primary enzyme oxidizes primary alcohols, including up to heptanol, at rates similar to that of ethanol. It is inactive with secondary alcohols. The secondary enzyme is inactive with 1-pentanol or longer chain alcohols. Its best substrate is 2-propanol, which is oxidized 15 times faster than ethanol. The secondary alcohol dehydrogenase is formed early during the growth cycle. It is stimulated by pyruvate and has a low Km for acetaldehyde (44.8 mM) in comparison to that of the primary alcohol dehydrogenase (210 mM). The latter enzyme is formed late in the growth cycle. It is postulated that the secondary alcohol dehydrogenase is largely responsible for the formation of ethanol in fermentations of carbohydrates by T. ethanolicus.
Full text
PDF

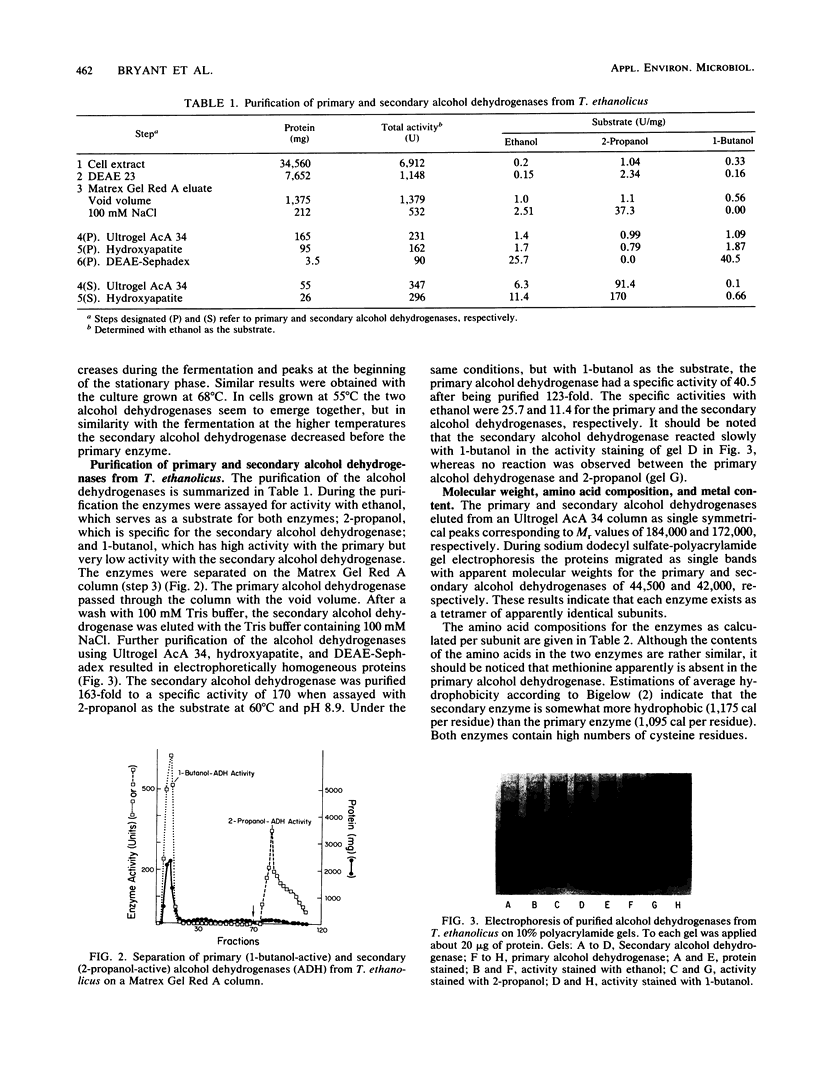
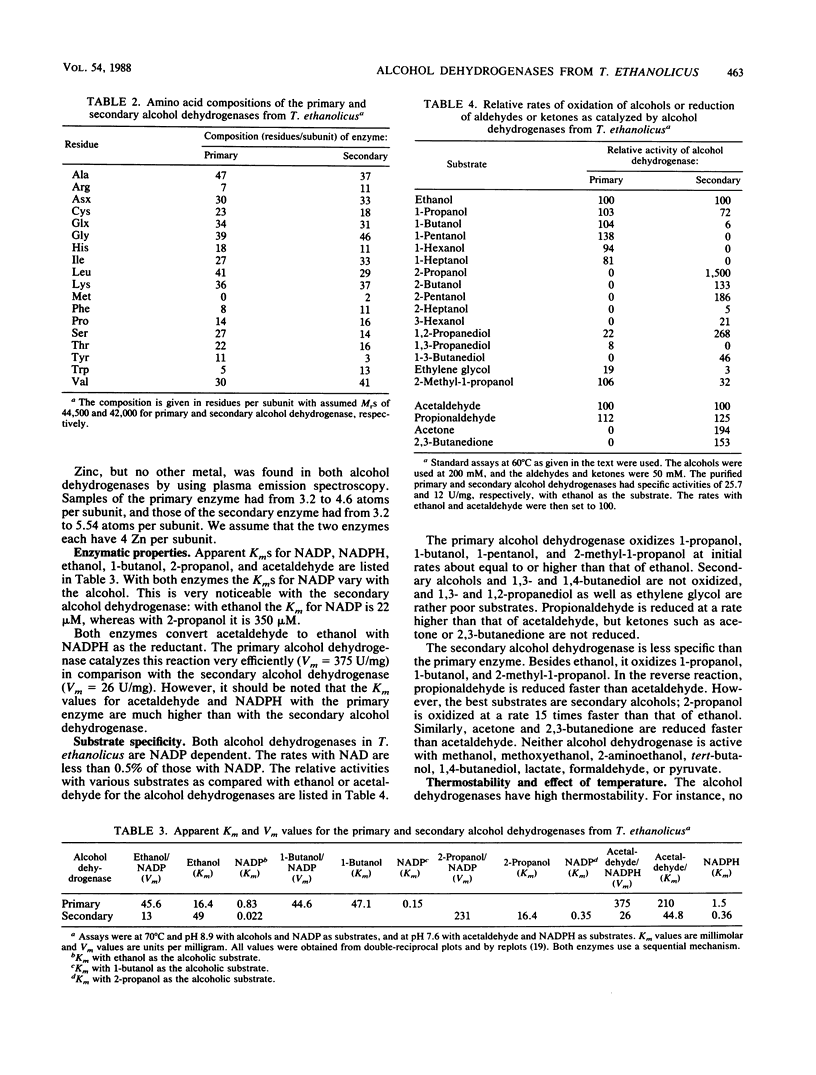
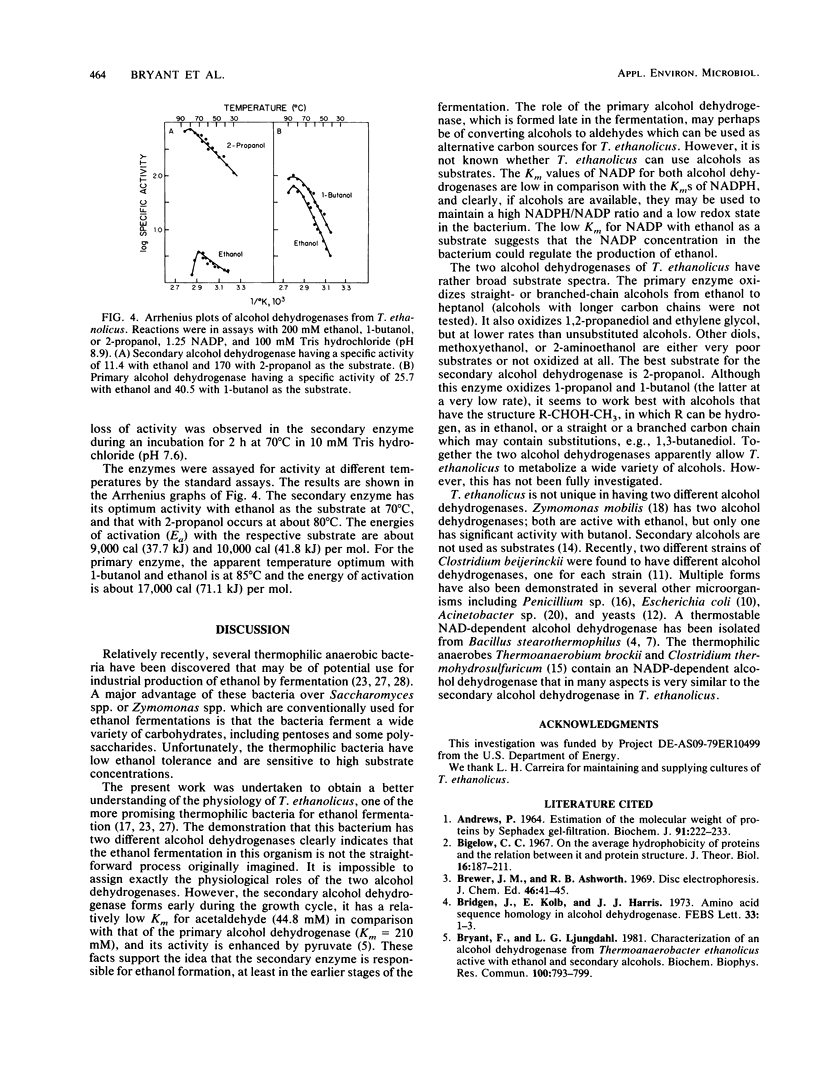

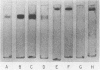
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow C. C. On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol. 1967 Aug;16(2):187–211. doi: 10.1016/0022-5193(67)90004-5. [DOI] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Kolb E., Harris J. I. Amino acid sequence homology in alcohol dehydrogenase. FEBS Lett. 1973 Jun 15;33(1):1–3. doi: 10.1016/0014-5793(73)80144-9. [DOI] [PubMed] [Google Scholar]
- Bryant F., Ljungdahl L. G. Characterization of an alcohol, dehydrogenase from Thermoanaerobacter ethanolicus active with ethanol and secondary alcohols. Biochem Biophys Res Commun. 1981 May 29;100(2):793–799. doi: 10.1016/s0006-291x(81)80244-6. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Comer M. J., Craven D. B., Harvey M. J., Atkinson A., Dean P. D. Affinity chromatography on immobilised nucleotides. Some applications to the purification of thermophilic dehydrogenases and kinases. Eur J Biochem. 1975 Jun 16;55(1):201–209. doi: 10.1111/j.1432-1033.1975.tb02152.x. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Brewer J. M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978 Sep;190(1):351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- Hiu S. F., Zhu C. X., Yan R. T., Chen J. S. Butanol-Ethanol Dehydrogenase and Butanol-Ethanol-Isopropanol Dehydrogenase: Different Alcohol Dehydrogenases in Two Strains of Clostridium beijerinckii (Clostridium butylicum). Appl Environ Microbiol. 1987 Apr;53(4):697–703. doi: 10.1128/aem.53.4.697-703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Marczak I. Substrate specificity and stereospecificity of nicotinamide adenine dinucleotide-linked alcohol dehydrogenases from methanol-grown yeasts. Appl Environ Microbiol. 1981 Mar;41(3):829–832. doi: 10.1128/aem.41.3.829-832.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston-Unkefer P. J., Gander J. E. Occurrence of multiple forms of alcohol dehydrogenase in Penicillium supplemented with 2,3-butanediol. Arch Biochem Biophys. 1984 Sep;233(2):447–456. doi: 10.1016/0003-9861(84)90466-1. [DOI] [PubMed] [Google Scholar]
- Neale A. D., Scopes R. K., Kelly J. M., Wettenhall R. E. The two alcohol dehydrogenases of Zymomonas mobilis. Purification by differential dye ligand chromatography, molecular characterisation and physiological roles. Eur J Biochem. 1986 Jan 2;154(1):119–124. doi: 10.1111/j.1432-1033.1986.tb09366.x. [DOI] [PubMed] [Google Scholar]
- Singer M. E., Finnerty W. R. Alcohol dehydrogenases in Acinetobacter sp. strain HO1-N: role in hexadecane and hexadecanol metabolism. J Bacteriol. 1985 Dec;164(3):1017–1024. doi: 10.1128/jb.164.3.1017-1024.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursprung H., Leone J. Alcohol dehydrogenases: a polymorphism in Drosophila melanogaster. J Exp Zool. 1965 Nov;160(2):147–154. doi: 10.1002/jez.1401600202. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]