Abstract
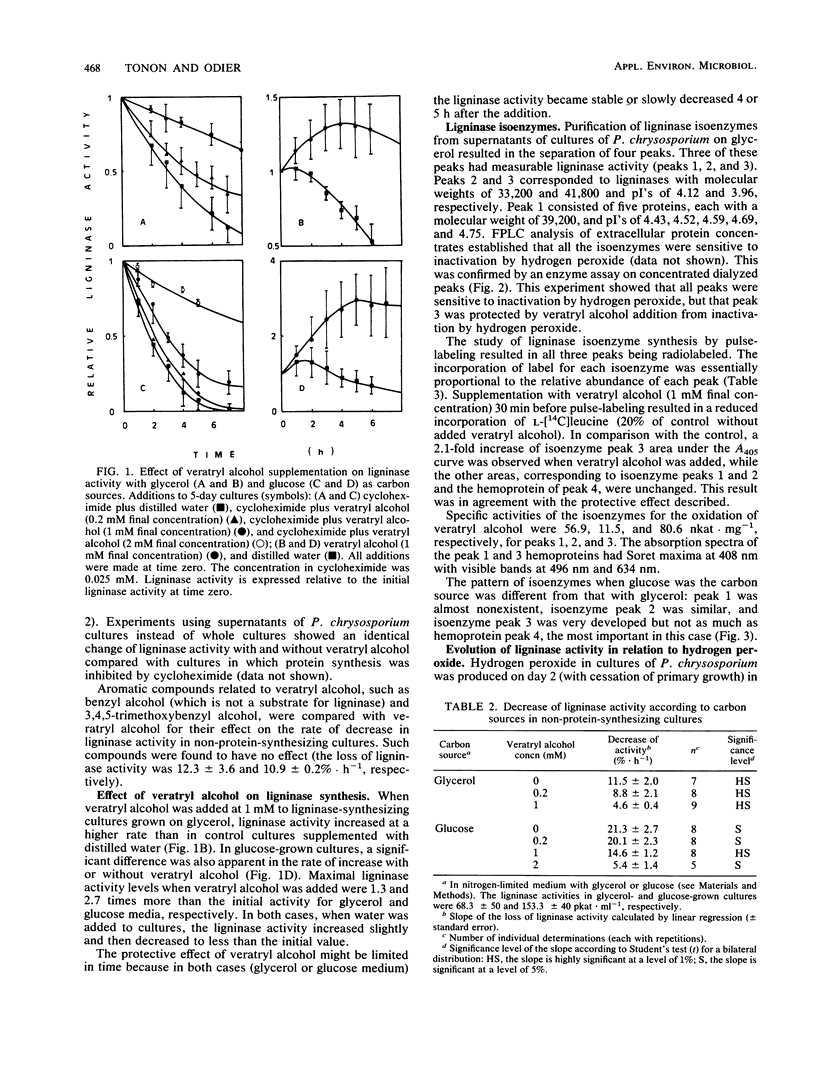
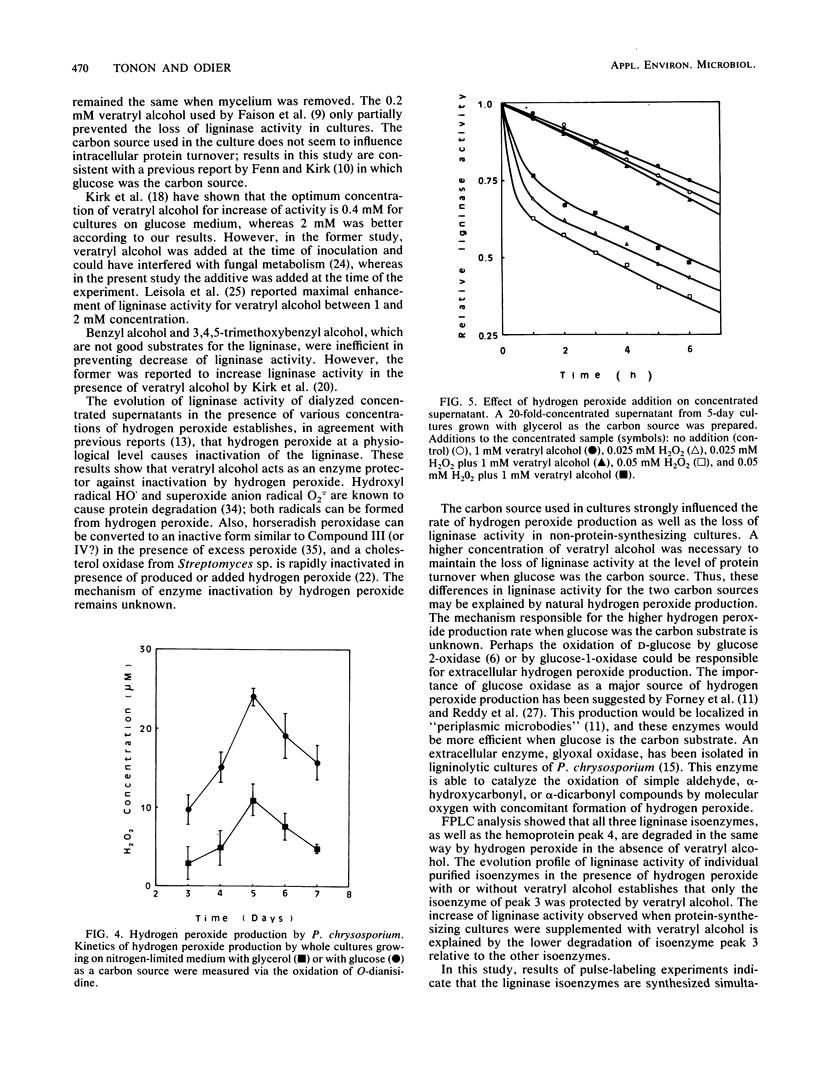
Veratryl alcohol, added as a supplement to cultures of Phanerochaete chrysosporium, enhanced ligninase activity through protection of the ligninase against inactivation by hydrogen peroxide produced by this fungus in cultures. In the presence of veratryl alcohol, the loss of ligninase activity observed in non-protein-synthesizing cultures (cycloheximide-treated) equaled the extracellular protein turnover. When cultures were not supplemented with veratryl alcohol, inactivation of ligninase by hydrogen peroxide added to protein turnover, resulting in a more rapid loss of ligninase activity. Although all ligninase isoenzymes are sensitive to inactivation by hydrogen peroxide, only the isoenzyme of the highest specific activity (80.6 nkat · mg of protein−1; Mr, 41,800; pI, 3.96) was found to be protected by veratryl alcohol. The concentration of veratryl alcohol necessary for full protection of ligninase activity varied according to the concentration of hydrogen peroxide present in the medium, which depended on the nature of the carbon source (glucose or glycerol). It is proposed that the nature of the carbon source influences the overall ligninase activity not only directly, by affecting the rate and the type of synthesized ligninase, but also by affecting the rate of hydrogen peroxide production, bringing about different rates of inactivation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Relationship Between Lignin Degradation and Production of Reduced Oxygen Species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Nov;46(5):1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Tien M., Kalyanaraman B., Kirk T. K. The ligninase of Phanerochaete chrysosporium generates cation radicals from methoxybenzenes. J Biol Chem. 1985 Mar 10;260(5):2609–2612. [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan V., Miki K., Gold M. H. Multiple molecular forms of diarylpropane oxygenase, an H2O2-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Aug 15;241(1):304–314. doi: 10.1016/0003-9861(85)90387-x. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]


