Abstract
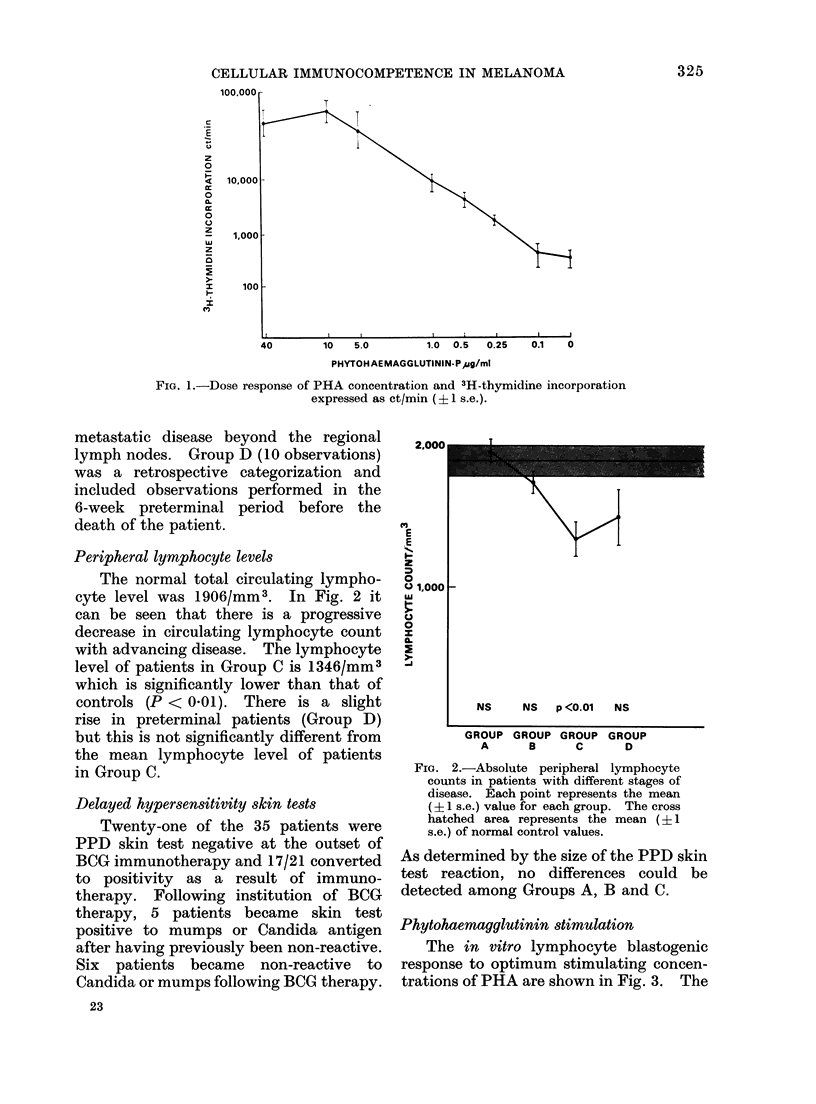
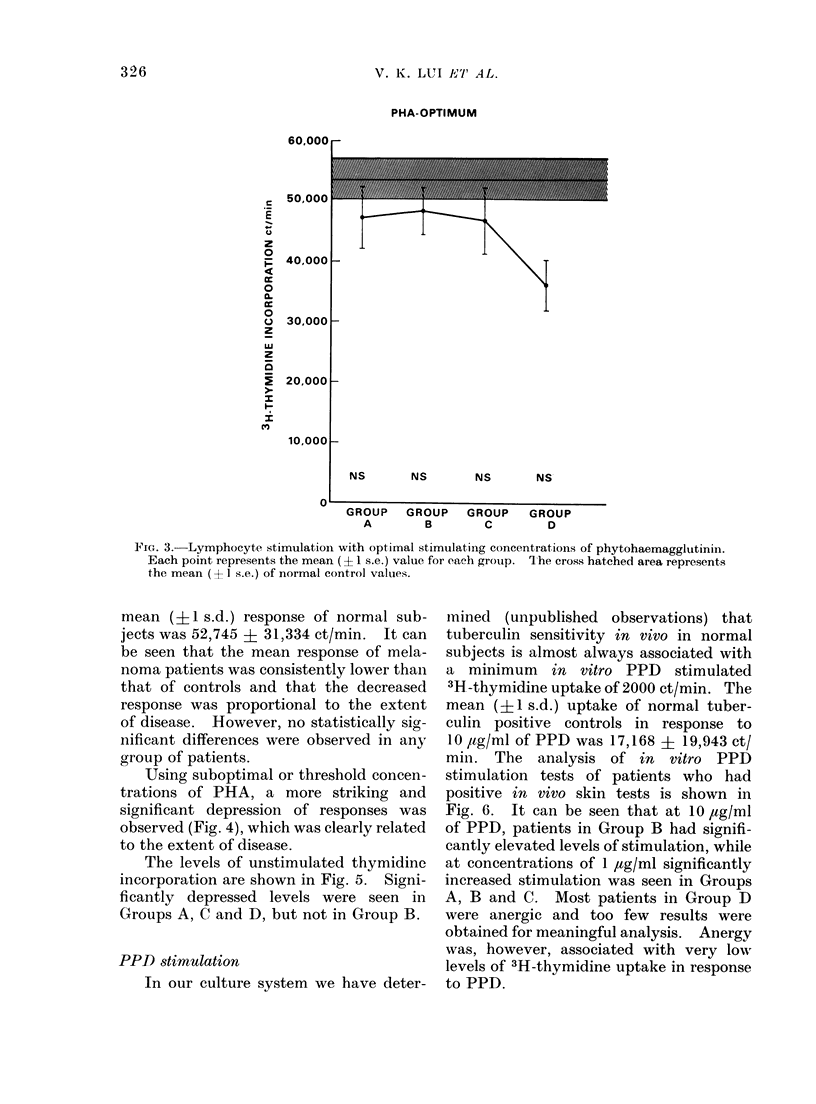
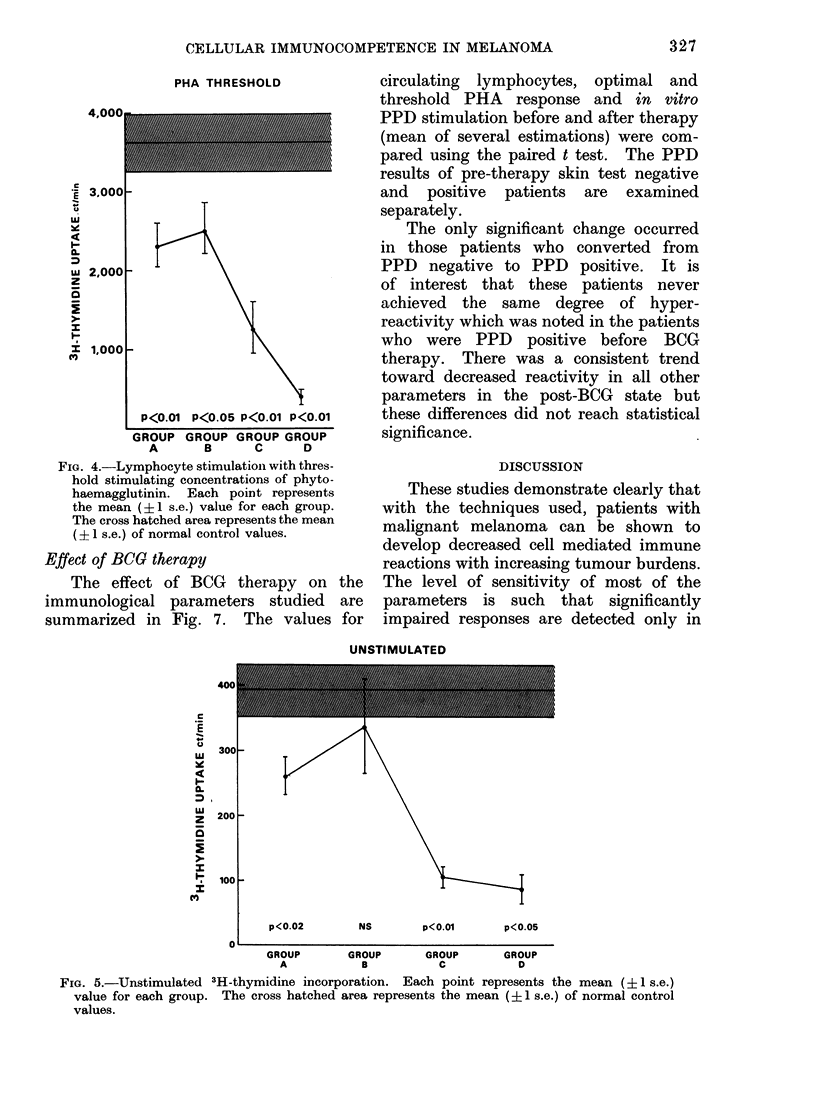
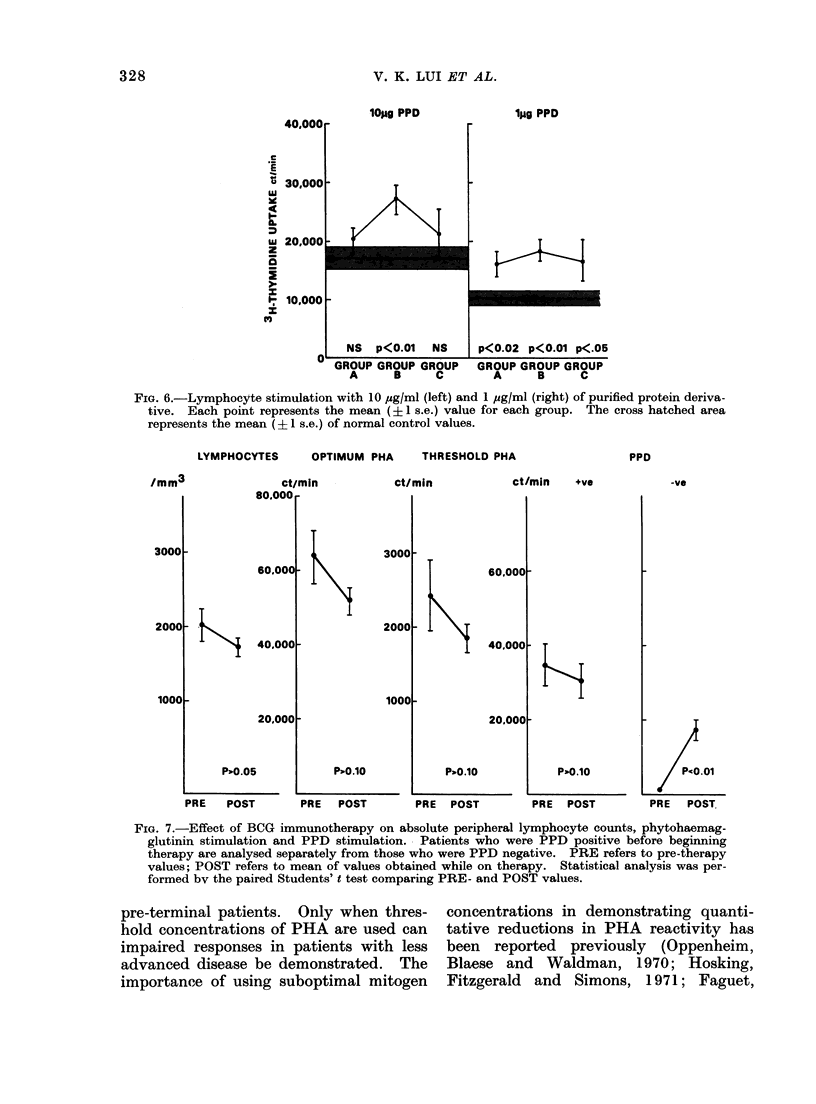
Cell mediated immunocompetence was measured serially in 35 patients with malignant melanoma in order to determine the effect of extent of disease and prognosis as well as the influence of BCG immunotherapy on immune reactivity. Compared with normal adult controls, statistically significant lymphopenia occurred only in patients with widespread disease. Seventeen of 21 patients with negative pre-therapy PPD skin test converted to skin test positivity. PHA blastogenesis was depressed only in patients in the pre-terminal stages of their disease using optimal mitogen concentrations for stimulation. Threshold concentrations of this mitogen more clearly demonstrated a depressed responsiveness which correlated in severity with extent of disease. PPD induced blastogenesis was normal or increased in the majority of patients; however, the degree of stimulation by PPD was less in the BCG induced convertors than in those patients who were skin test positive before BCG treatment. Comparison of the pre- and post BCG assessments reveals no significant differences except in relation to PPD conversion. We conclude that using threshold concentrations of PHA, impaired responses are regularly associated with disease beyond the regional lymph nodes. Routine assessment of lymphocyte function by these parameters did not provide information that was not available from clinical evaluation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bluming A. Z., Vogel C. L., Ziegler J. L., Mody N., Kamya G. Immunological effects of BCG in malignant melanoma: two modes of administration compared. Ann Intern Med. 1972 Mar;76(3):405–411. doi: 10.7326/0003-4819-76-3-405. [DOI] [PubMed] [Google Scholar]
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Catalona W. J., Chretien P. B. Abnormalities of quantitative dinitrochlorobenzene sensitization in cancer patients: correlation with tumor stage and histology. Cancer. 1973 Feb;31(2):353–356. doi: 10.1002/1097-0142(197302)31:2<353::aid-cncr2820310213>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Catalona W. J., Taylor P. T., Rabson A. S., Chretien P. B. A method for dinitrochlorobenzene contact sensitization. A clinicopathological study. N Engl J Med. 1972 Feb 24;286(8):399–402. doi: 10.1056/NEJM197202242860804. [DOI] [PubMed] [Google Scholar]
- Chakravorty R. C., Curutchet H. P., Coppolla F. S., Park C. M., Blaylock W. K., Lawrence W., Jr The delayed hypersensitivity reaction in cancer patient: observations on sensitization by DNCB. Surgery. 1973 May;73(5):730–735. [PubMed] [Google Scholar]
- Chess L., Bock G. N., Ungaro P. C., Buchholz D. H., Mardiney M. R., Jr Immunologic effects of BCG in patients with malignant melanoma: specific evidence for stimulation of the 'secondary' immune response. J Natl Cancer Inst. 1973 Jul;51(1):57–65. doi: 10.1093/jnci/51.1.57. [DOI] [PubMed] [Google Scholar]
- Dent P. B. Inhibition by phytohemagglutinin of DNA synthesis in cultured mouse lymphomas. J Natl Cancer Inst. 1971 Apr;46(4):763–773. [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Golub S. H., O'Connell T. X., Morton D. L. Correlation of in vivo and in vitro assays of immunocompetence in cancer patients. Cancer Res. 1974 Aug;34(8):1833–1837. [PubMed] [Google Scholar]
- Greene W. H., Schimpff S. C., Wiernik P. H. Cell-mediated immunity in acute nonlymphocytic leukemia: relationship to host factors, therapy, and prognosis. Blood. 1974 Jan;43(1):1–14. [PubMed] [Google Scholar]
- Gutterman J., Mavligit G., McBride C., Frei E., 3rd, Hersh E. M. BCG stimulation of immune responsiveness in patients with malignant melanoma. Preliminary report. Cancer. 1973 Aug;32(2):321–327. doi: 10.1002/1097-0142(197308)32:2<321::aid-cncr2820320207>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Harris J., Copeland D. Impaired immunoresponsiveness in tumor patients. Ann N Y Acad Sci. 1974;230:56–85. doi: 10.1111/j.1749-6632.1974.tb14437.x. [DOI] [PubMed] [Google Scholar]
- Hosking C. S., Fitzgerald M. G., Simons M. J. Quantified deficiency of lymphocyte response to phytohaemagglutinin in immune deficiency diseases. Clin Exp Immunol. 1971 Oct;9(4):467–476. [PMC free article] [PubMed] [Google Scholar]
- Junge U., Hoekstra J., Wolfe L., Deinhardt F. Microtechnique for quantitative evaluation of in vitro lymphocyte transformation. Clin Exp Immunol. 1970 Sep;7(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Blaese R. M., Waldmann T. A. Defective lymphocyte transformation and delayed hypersensitivity in Wiskott-Aldrich syndrome. J Immunol. 1970 Apr;104(4):835–844. [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Seiger H. F., Shingleton W. W., Metzgar R. S., Buckley C. E., 3rd Immunotherapy in patients with melanoma. Ann Surg. 1973 Sep;178(3):352–359. doi: 10.1097/00000658-197309000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal J. E., Aungst C. W., Han T. Effect of BCG on delayed hypersensitivity responses of patients with neoplastic disease. Int J Cancer. 1973 Jul 15;12(1):242–249. doi: 10.1002/ijc.2910120125. [DOI] [PubMed] [Google Scholar]
- Ziegler J. L., Lewis M. G., Luyombya J. M., Kiryabwire J. W. Immunologic studies in patients with malignant melanoma in Uganda. Br J Cancer. 1969 Dec;23(4):729–734. doi: 10.1038/bjc.1969.89. [DOI] [PMC free article] [PubMed] [Google Scholar]


