Abstract
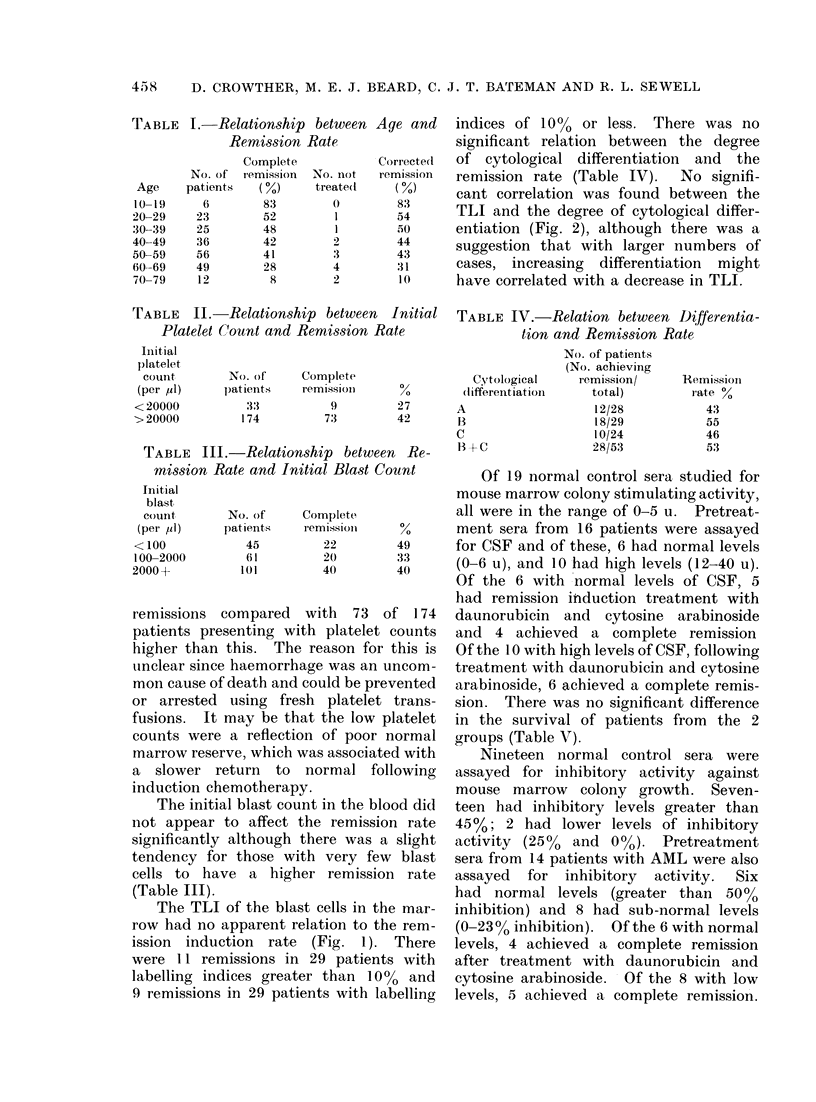
A study of the thymidine labelling index (TLI) of bone marrow blast cells in 58 untreated patients with acute myelogenous leukemia showed no correlation with remission rate but there was a strong correlation between labelling index and remission length in the 21 patients who achieved remission. The median remission length of the patients was 33 weeks. Of the 12 patients with initial labelling indices greater than 10%, only 2 had remissions longer than 33 weeks whereas 8 of the 9 patients with labelling indices less than 10% had remissions longer than 33 weeks. No correlation could be found between the degree of cytological differentiation and remission induction, remission length or survival. No correlation was found between the TLI and the degree of cytological differentiation. Age and initial platelet count were confirmed to be important factors influencing complete remission rate, but these factors did not correlate with remission length. Sixteen patients had their pretreatment sera assayed for mouse marrow colony stimulating activity and inhibitor levels but there was no correlation with subsequent response to treatment, although the number of patients examined was clearly too small for any definite conclusions to be drawn.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill F., Pindar A., Crowther D. Factors influencing microculture in leukaemia cells. Nature. 1974 Oct 25;251(5477):741–742. doi: 10.1038/251741a0. [DOI] [PubMed] [Google Scholar]
- Bernard J., Weil M., Boiron M., Jacquillat C., Flandrin G., Gemon M. F. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973 Apr;41(4):489–496. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Brincker H. Clinical classification and evaluation of treatment response in acute myeloid leukaemia on the basis of differences of leukaemic cell differentiation. Scand J Haematol. 1973;11(5):383–390. doi: 10.1111/j.1600-0609.1973.tb00148.x. [DOI] [PubMed] [Google Scholar]
- Burke P. J., Owens A. H., Jr Attempted recruitment of leukemic myeloblasts to proliferative activity by sequential drug treatment. Cancer. 1971 Oct;28(4):830–836. doi: 10.1002/1097-0142(1971)28:4<830::aid-cncr2820280405>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Clarkson B. D. Acute myelocytic leukemia in adults. Cancer. 1972 Dec;30(6):1572–1582. doi: 10.1002/1097-0142(197212)30:6<1572::aid-cncr2820300624>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Crowther D., Bateman C. J., Vartan C. P., Whitehouse J. M., Malpas J. S., Fairley G. H., Scott R. B. Combination chemotherapy using L-asparaginase, daunorubicin, and cytosine arabinoside in adults with acute myelogenous leukaemia. Br Med J. 1970 Nov 28;4(5734):513–517. doi: 10.1136/bmj.4.5734.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther D. Intensive treatment of acute leukaemia. Br J Clin Pract. 1971 Jun;25(6):271–277. [PubMed] [Google Scholar]
- Crowther D., Powles R. L., Bateman C. J., Beard M. E., Gauci C. L., Wrigley P. F., Malpas J. S., Fairley G. H., Scott R. B. Management of adult acute myelogenous leukaemia. Br Med J. 1973 Jan 20;1(5846):131–137. doi: 10.1136/bmj.1.5846.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Chan S. H., Gunz F. W., Vincent P., Ravich R. B. Colony-stimulating factor and inhibitor levels in acute granulocytic leukemia. Blood. 1971 Aug;38(2):143–152. [PubMed] [Google Scholar]
- Metcalf D., Moore M. A., Sheridan J. W., Spitzer G. Responsiveness of human granulocytic leukemic cells to colony-stimulating factor. Blood. 1974 Jun;43(6):847–859. [PubMed] [Google Scholar]
- Moore M. A., Williams N. Physical separation of colony stimulating cells from in vitro colony forming cells in hemopoietic tissue. J Cell Physiol. 1972 Oct;80(2):195–206. doi: 10.1002/jcp.1040800206. [DOI] [PubMed] [Google Scholar]
- Powles R. L., Crowther D., Bateman C. J., Beard M. E., McElwain T. J., Russell J., Lister T. A., Whitehouse J. M., Wrigley P. F., Pike M. Immunotherapy for acute myelogenous leukaemia. Br J Cancer. 1973 Nov;28(5):365–376. doi: 10.1038/bjc.1973.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. A., Pike B. L. Leukopoietic activity in human urine. The granulocytic leukemias. N Engl J Med. 1970 Jun 4;282(23):1291–1297. doi: 10.1056/NEJM197006042822304. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D., Maritz J. S., Yeo G. F. Standardized bioassay for bone marrow colony stimulating factor in human urine: levels in normal man. J Lab Clin Med. 1972 Apr;79(4):657–668. [PubMed] [Google Scholar]
- Vogler W. R., Cooper L. E., Groth D. P. Correlation of cytosine arabinoside-induced increment in growth fraction of leukemic blast cells with clinical response. Cancer. 1974 Mar;33(3):603–610. doi: 10.1002/1097-0142(197403)33:3<603::aid-cncr2820330302>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


