Abstract
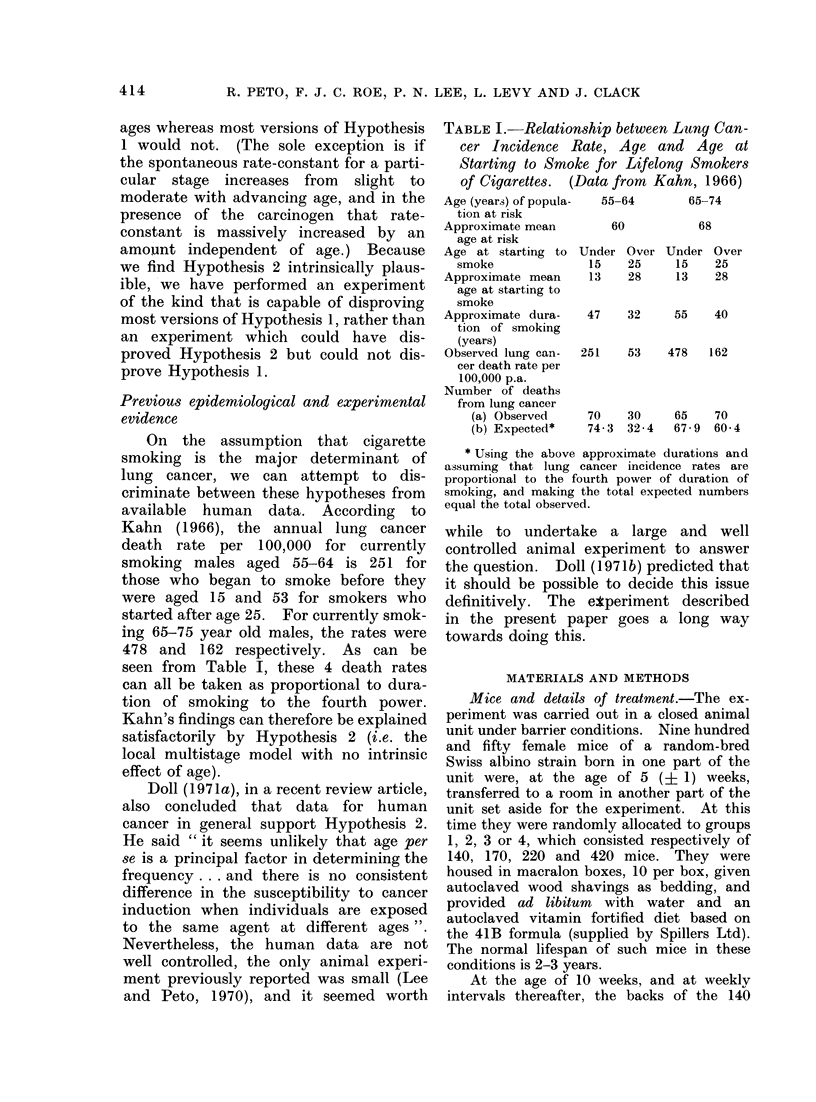
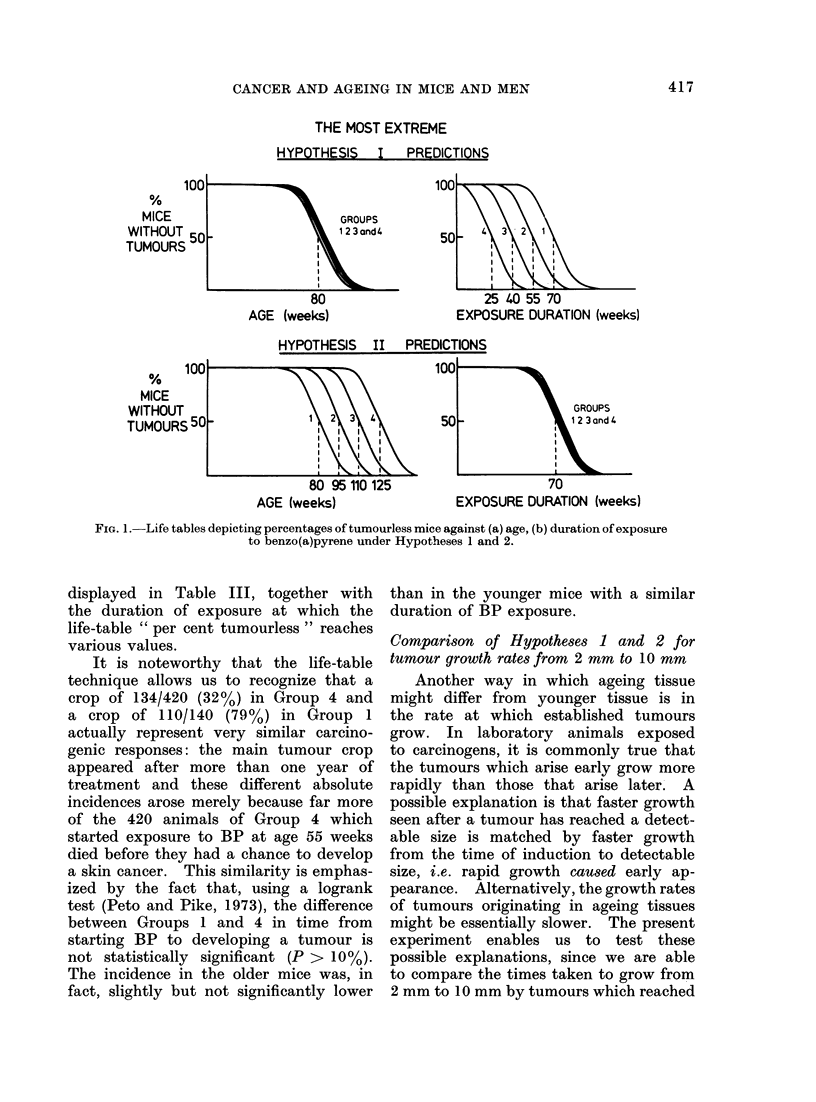
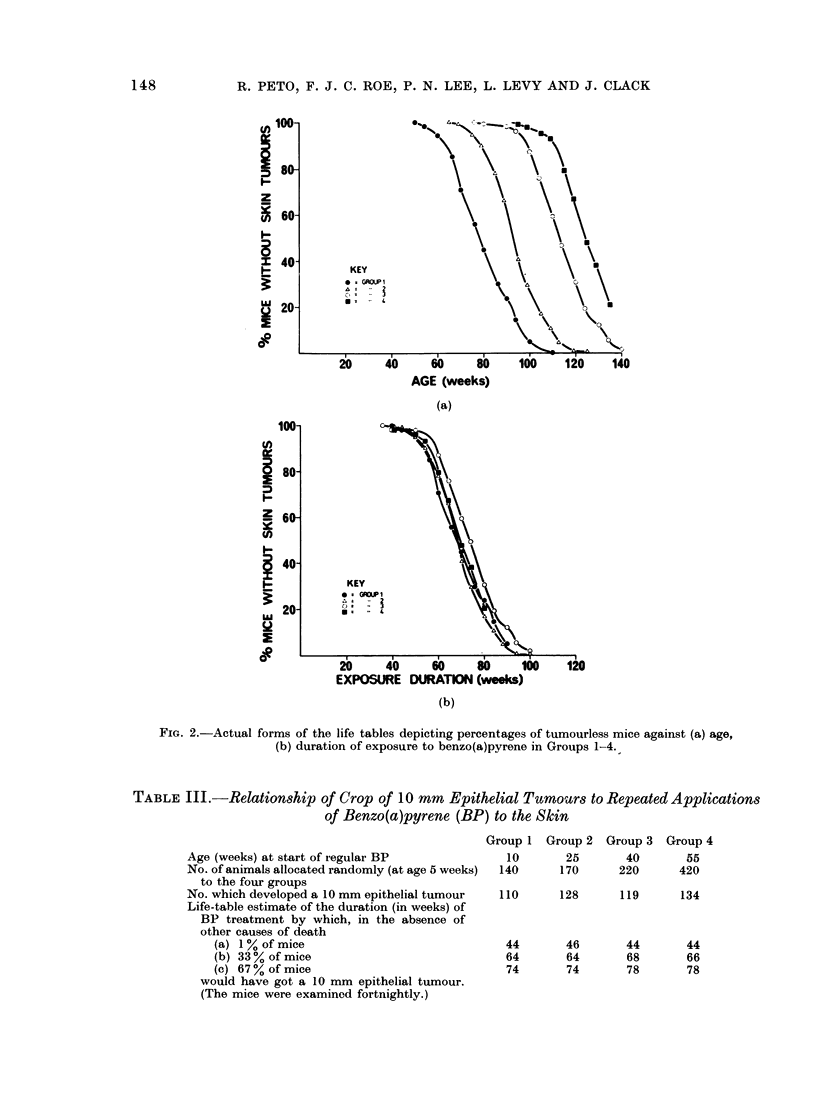
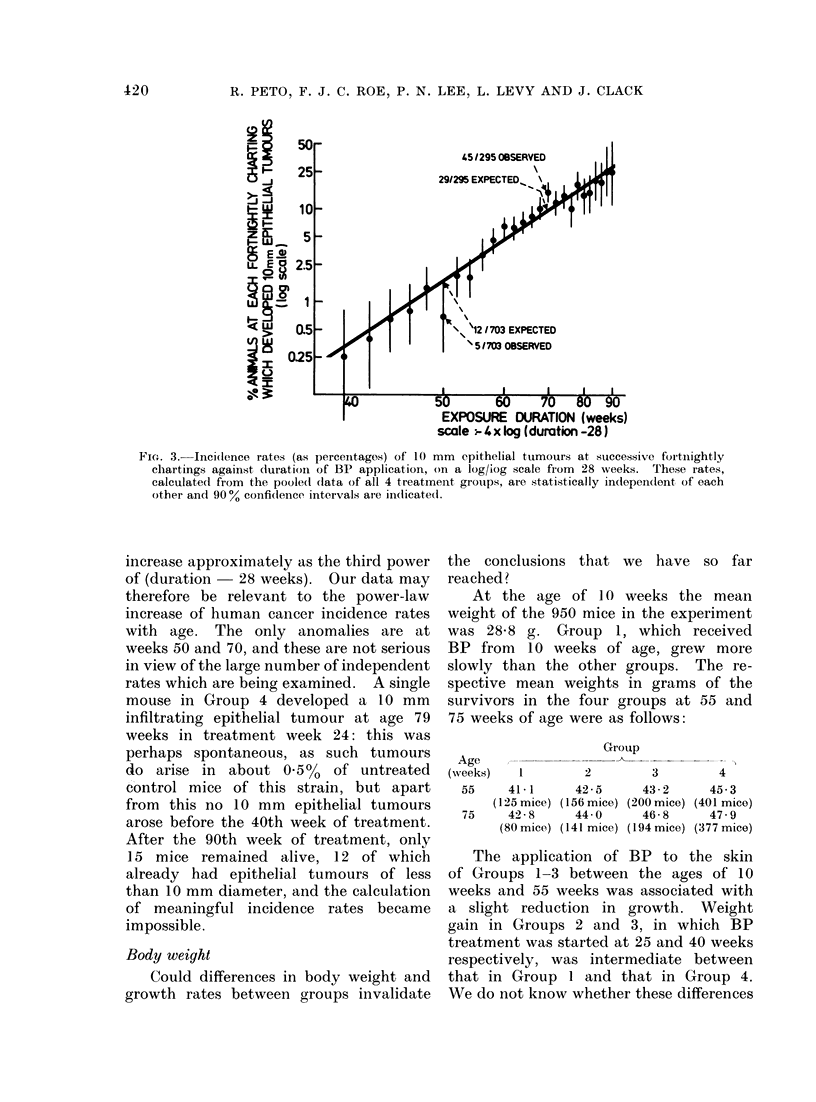
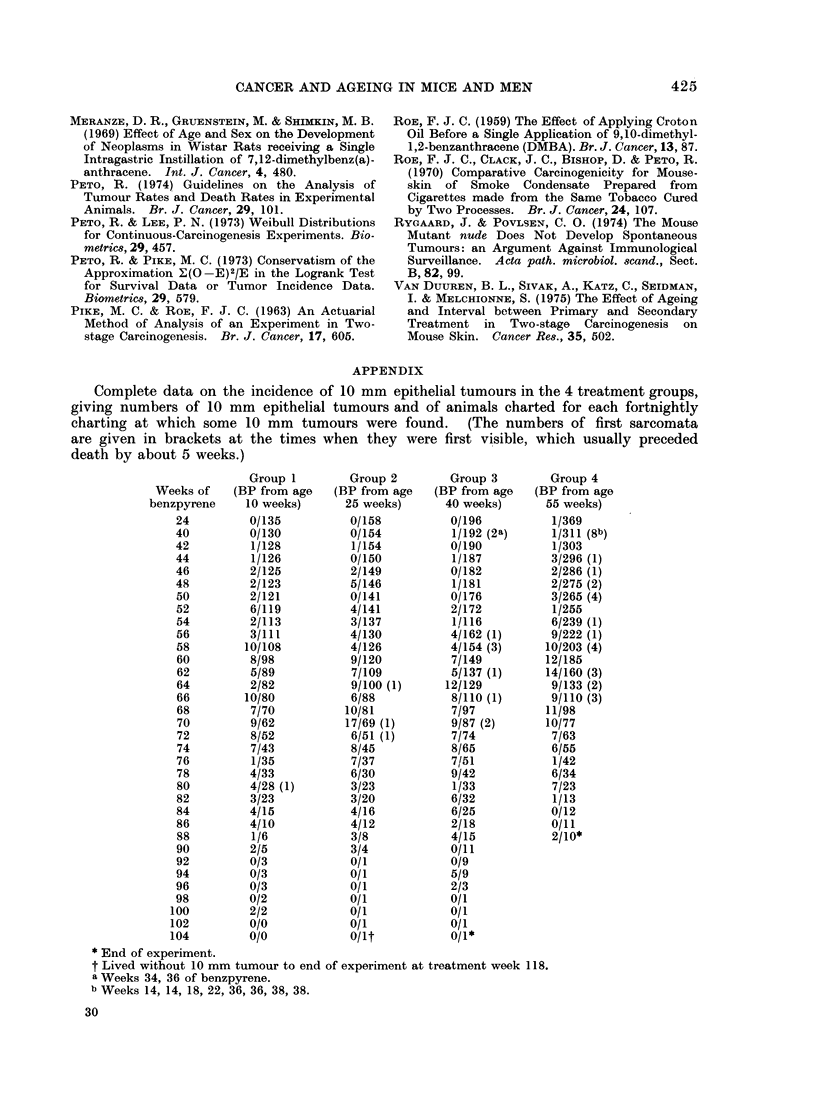
In an experiment involving 950 mice with a normal lifespan of 2-3 years, in laboratory conditions, regular benzpyrene application to the skin was started at 10, 25, 40 or 55 weeks of age. The incidence rate of malignant epithelial tumours among the survivors in each group increased steeply with time. This increase was associated directly with duration of exposure but, given duration, was independent of age at the start of exposure, as were the growth rates of already established tumours. In our experiment, although age per se was irrelevant, the cancer incidence rate increased approximately as a power of the duration of exposure to benzpyrene. This shows that the observed approximate power-law increase of most human adult cancer incidence rates with age could exist merely because age equals duration of exposure to background and spontaneous carcinogenic stimuli. Thus, no intrinsic effects of ageing (such as failing immunological surveillance or age related hormonal changes) whatever need to postulated to explain the vast increases in old age of the incidence rates of such human cancers. This result can greatly simplify speculation about mechanisms of carcinogenesis.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnet F. M. Intrinsic mutagenesis, an interpretation of the pathogenesis of xeroderma pigmentosum. Lancet. 1974 Aug 31;2(7879):495–498. doi: 10.1016/s0140-6736(74)92019-4. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Dilman V. M. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageine, and disease. Lancet. 1971 Jun 12;1(7711):1211–1219. doi: 10.1016/s0140-6736(71)91721-1. [DOI] [PubMed] [Google Scholar]
- Ebbesen P. Aging increases susceptibility of mouse skin to DMBA carcinogenesis independent of general immune status. Science. 1974 Jan 18;183(4121):217–218. doi: 10.1126/science.183.4121.217. [DOI] [PubMed] [Google Scholar]
- Ebbesen P. Papilloma induction in different aged skin grafts to young recipients. Nature. 1973 Jan 26;241(5387):280–281. doi: 10.1038/241280a0. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Lee P. N., O'Neill J. A. The effect both of time and dose applied on tumour incidence rate in benzopyrene skin painting experiments. Br J Cancer. 1971 Dec;25(4):759–770. doi: 10.1038/bjc.1971.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. N., Peto R. The effect of the age of mice on the incidence of skin cancer. Br J Cancer. 1970 Dec;24(4):849–852. doi: 10.1038/bjc.1970.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meranze D. R., Gruenstein M., Shimkin M. B. Effect of age and sex on the development of neoplasms in wistar rats receiving a single intragastric instillation of 7,13-Dimethylbenz(a)anthracene. Int J Cancer. 1969 Jul 15;4(4):480–486. doi: 10.1002/ijc.2910040413. [DOI] [PubMed] [Google Scholar]
- PIKE M. C., ROE F. J. AN ACTUARIAL METHOD OF ANALYSIS OF AN EXPERIMENT IN TWO-STAGE CARCINOGENESIS. Br J Cancer. 1963 Dec;17:605–610. doi: 10.1038/bjc.1963.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R. Editorial: Guidelines on the analysis of tumour rates and death rates in experimental animals. Br J Cancer. 1974 Feb;29(2):101–105. doi: 10.1038/bjc.1974.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Lee P. Weibull distributions for continuous-carcinogenesis experiments. Biometrics. 1973 Sep;29(3):457–470. [PubMed] [Google Scholar]
- Peto R., Pike M. C. Conservatism of the approximation sigma (O-E)2-E in the logrank test for survival data or tumor incidence data. Biometrics. 1973 Sep;29(3):579–584. [PubMed] [Google Scholar]
- ROE F. J. The effect of applying croton oil before a single application of 9,10-dimathyl-1,2-benzanthracene. Br J Cancer. 1959 Mar;13(1):87–91. doi: 10.1038/bjc.1959.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe F. J., Clack J. C., Bishop D., Peto R. Comparative carcinogenicity for mouse-skin of smoke condensates prepared from cigarettes made from the same tobacco cured by two processes. Br J Cancer. 1970 Mar;24(1):107–121. doi: 10.1038/bjc.1970.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duuren B. L., Sivak A., Katz C., Seidman I., Melchionne S. The effect of aging and interval between primary and secondary treatment in two-stage carcinogenesis on mouse skin. Cancer Res. 1975 Mar;35(3):502–505. [PubMed] [Google Scholar]


