Abstract
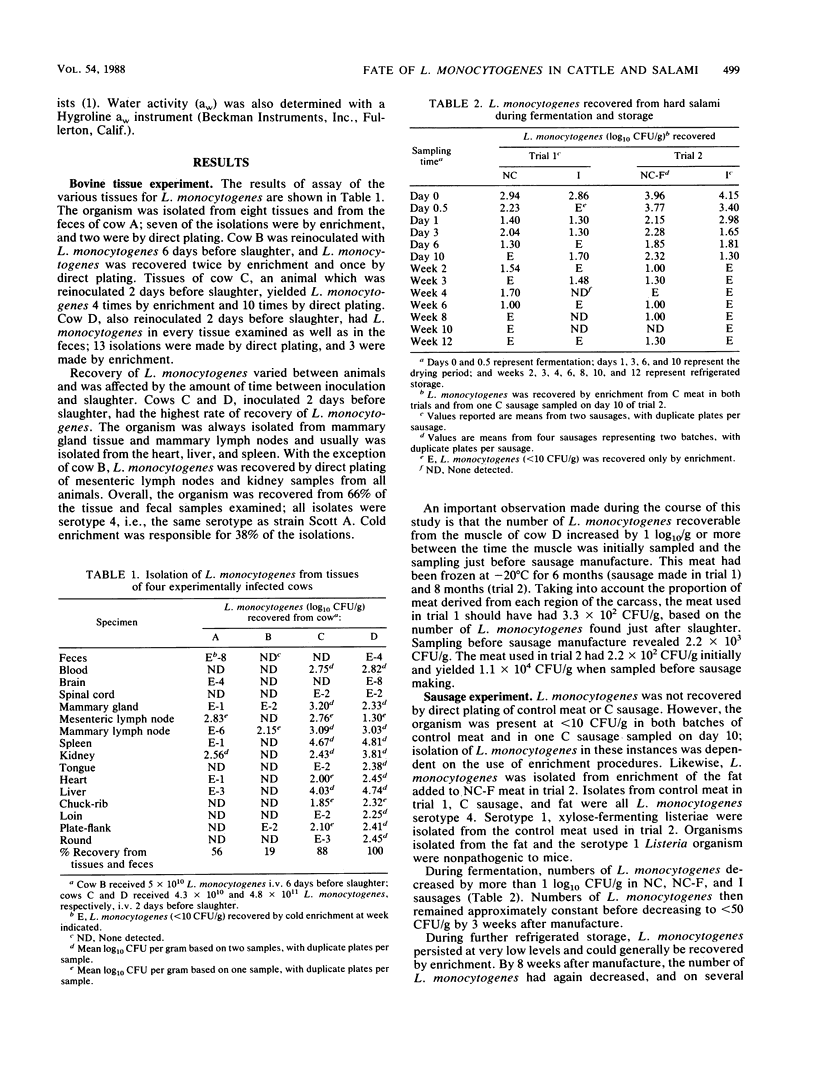
Muscle, organ, and lymphoid tissues of four Holstein cows experimentally inoculated (intravenously) with Listeria monocytogenes were examined 2, 6, or 54 days postinoculation for the presence of the organism by direct plating and cold enrichment procedures. L. monocytogenes was isolated from 66% of the tissues sampled; 38% of the isolations were attributed to the use of cold enrichment. Isolation of the organism from muscle tissue was possible only with animals inoculated 2 days before slaughter. The fate of L. monocytogenes during the manufacture and storage of fermented hard salami made from this meat also was determined. Three sausage treatments were evaluated: (i) uninoculated control sausage, (ii) "naturally" contaminated sausage (NC) made from meat of an experimentally inoculated cow, and (iii) sausage made from beef inoculated with a laboratory culture of L. monocytogenes (I). Initial Listeria levels in NC and I sausage were 10(3) CFU/g in trial 1 and 10(4) CFU/g in trial 2. Numbers of L. monocytogenes decreased by approximately 1 log10 CFU/g during fermentation and decreased further during drying and refrigerated storage. Small numbers (less than or equal to 20 CFU/g) of L. monocytogenes were present in I and NC sausage at the end of 12 weeks of refrigerated storage; recovery of these organisms generally depended on the use of an enrichment procedure. The results indicate that L. monocytogenes does not multiply during the fermentation and drying processes typical of hard salami manufacture but that survival may occur if the organism is initially present at greater than or equal to 10(3) CFU/g.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conner D. E., Brackett R. E., Beuchat L. R. Effect of temperature, sodium chloride, and pH on growth of Listeria monocytogenes in cabbage juice. Appl Environ Microbiol. 1986 Jul;52(1):59–63. doi: 10.1128/aem.52.1.59-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. W., Baigent G. J. Method for flow cytometric detection of Listeria monocytogenes in milk. Appl Environ Microbiol. 1986 Oct;52(4):689–695. doi: 10.1128/aem.52.4.689-695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P., Glass K. A., Beery J. T., Garcia G. A., Pollard D. J., Schultz R. D. Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl Environ Microbiol. 1987 Jul;53(7):1433–1438. doi: 10.1128/aem.53.7.1433-1438.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Stafseth H. J., Thorp F., Sholl L. B., Riley W. F. A New Technique for Isolating Listerellae from the Bovine Brain. J Bacteriol. 1948 Apr;55(4):471–476. [PMC free article] [PubMed] [Google Scholar]
- KAUTTER D. A., SILVERMAN S. J., ROESSLER W. G., DRAWDY J. F. Virulence of Listeria monocytogenes for experimental animals. J Infect Dis. 1963 Mar-Apr;112:167–180. doi: 10.1093/infdis/112.2.167. [DOI] [PubMed] [Google Scholar]
- Lee W. H., McClain D. Improved Listeria monocytogenes selective agar. Appl Environ Microbiol. 1986 Nov;52(5):1215–1217. doi: 10.1128/aem.52.5.1215-1217.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLDING L., PHILIPSON L. Two cases of listeriosis in the newborn, associated with placental infection. Acta Pathol Microbiol Scand. 1960;48:24–30. doi: 10.1111/j.1699-0463.1960.tb04731.x. [DOI] [PubMed] [Google Scholar]
- Pittman B., Cherry W. B. Isolation of Listeria monocytogenes from brains of rabies-negative animals. Am J Vet Res. 1967 May;28(124):779–785. [PubMed] [Google Scholar]
- Wilkins P. O., Bourgeois R., Murray R. G. Psychrotrophic properties of Listeria monocytogenes. Can J Microbiol. 1972 May;18(5):543–551. doi: 10.1139/m72-087. [DOI] [PubMed] [Google Scholar]
- Wilkinson T. R., Hall E. R. Rapid method for the isolation of Listeria monocytogenes from experimentally infected mice. Appl Microbiol. 1971 Jan;21(1):112–118. doi: 10.1128/am.21.1.112-118.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINK A., de MELLO G. C., BURKHART R. L. Listeriosis; field and laboratory studies, and aureomycin activity. Am J Vet Res. 1951 Jul;12(44):194–198. [PubMed] [Google Scholar]


