Abstract
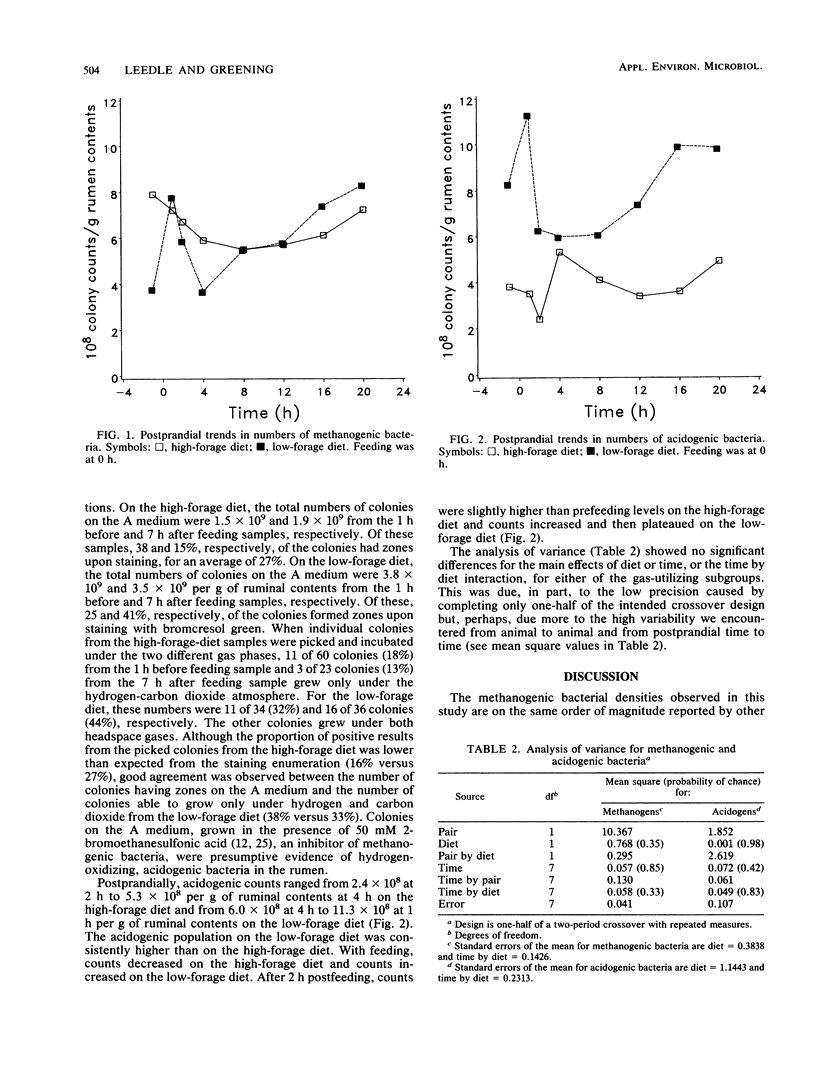
Four ruminally fistulated Hereford steers (400 kg) were fed two isocaloric diets at 1.5 x maintenance once daily in a repeated measurement crossover experiment. Postprandial changes in hydrogen-oxidizing, carbon dioxide-reducing bacterial groups were monitored. The methanogenic bacterial populations were present at densities of 4 x 10(8) to 8 x 10(8)/g of ruminal contents on either the high- or low-forage diet. Numbers remained constant postprandially on the high-forage diet but showed a distinct rise and fall with the once-daily feeding of the low-forage diet. Presumed hydrogen- and carbon dioxide-utilizing, acid-producing (acidogenic) bacteria were present between 2 x 10(8) and 12 x 10(8)/g of ruminal contents, with the density of the low-forage population being twofold higher than that of the high-forage population. Acidogenic bacteria exhibited similar postprandial changes on both diets, with the predominant shift being associated with the feeding event. This is the first study which documents the postfeeding trends in ruminal methanogenic bacteria on specified, production-level diets. It is also the first study to suggest that other hydrogen-oxidizing, carbon dioxide-reducing bacteria which produce acid instead of methane are present at high population densities in the normally fed adult ruminant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Iriki T., Tobe N., Shibui H. Sequestration of holotrich protozoa in the reticulo-rumen of cattle. Appl Environ Microbiol. 1981 Mar;41(3):758–765. doi: 10.1128/aem.41.3.758-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Schoberth S., Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979 Mar 12;120(3):201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Switzer J. M. Acetate Synthesis from H(2) plus CO(2) by Termite Gut Microbes. Appl Environ Microbiol. 1986 Oct;52(4):623–630. doi: 10.1128/aem.52.4.623-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Effects of diet, time after feeding, and position sampled on numbers of viable bacteria in the bovine rumen. J Dairy Sci. 1968 Dec;51(12):1950–1955. doi: 10.3168/jds.S0022-0302(68)87320-5. [DOI] [PubMed] [Google Scholar]
- Czerkawski J. W., Breckenridge G. Determination of concentration of hydrogen and some other cases dissolved in biological fluids. Lab Pract. 1971 May;20(5):403–passim. [PubMed] [Google Scholar]
- Czerkawski J. W. Methane production in ruminants and its significance. World Rev Nutr Diet. 1969;11:240–282. doi: 10.1159/000387580. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E., BRYANT M. P., MAH R. A. THE RUMEN BACTERIA AND PROTOZOA. Annu Rev Microbiol. 1964;18:131–166. doi: 10.1146/annurev.mi.18.100164.001023. [DOI] [PubMed] [Google Scholar]
- Hobson P. N., Wallace R. J. Microbial ecology and activities in the rumen: part 1. Crit Rev Microbiol. 1982 Apr;9(3):165–225. doi: 10.3109/10408418209104490. [DOI] [PubMed] [Google Scholar]
- Krumholz L. R., Forsberg C. W., Veira D. M. Association of methanogenic bacteria with rumen protozoa. Can J Microbiol. 1983 Jun;29(6):676–680. doi: 10.1139/m83-110. [DOI] [PubMed] [Google Scholar]
- Leedle J. A., Barsuhn K., Hespell R. B. Postprandial trends in estimated ruminal digesta polysaccharides and their relation to changes in bacterial groups and ruminal fluid characteristics. J Anim Sci. 1986 Mar;62(3):789–803. doi: 10.2527/jas1986.623789x. [DOI] [PubMed] [Google Scholar]
- Leedle J. A., Bryant M. P., Hespell R. B. Diurnal variations in bacterial numbers and fluid parameters in ruminal contents of animals fed low- or high-forage diets. Appl Environ Microbiol. 1982 Aug;44(2):402–412. doi: 10.1128/aem.44.2.402-412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedle J. A., Hespell R. B. Differential carbohydrate media and anaerobic replica plating techniques in delineating carbohydrate-utilizing subgroups in rumen bacterial populations. Appl Environ Microbiol. 1980 Apr;39(4):709–719. doi: 10.1128/aem.39.4.709-719.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser D. B., Moir R. J. Dietary effects upon concentrations of protozoa in the rumen. J Anim Sci. 1966 Aug;25(3):668–674. doi: 10.2527/jas1966.253668x. [DOI] [PubMed] [Google Scholar]
- Robinson J. A., Strayer R. F., Tiedje J. M. Method for measuring dissolved hydrogen in anaerobic ecosystems: application to the rumen. Appl Environ Microbiol. 1981 Feb;41(2):545–548. doi: 10.1128/aem.41.2.545-548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Anguish T., Cardwell S. C. Selective inhibition by 2-bromoethanesulfonate of methanogenesis from acetate in a thermophilic anaerobic digestor. Appl Environ Microbiol. 1984 Jun;47(6):1343–1345. doi: 10.1128/aem.47.6.1343-1345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


