Abstract
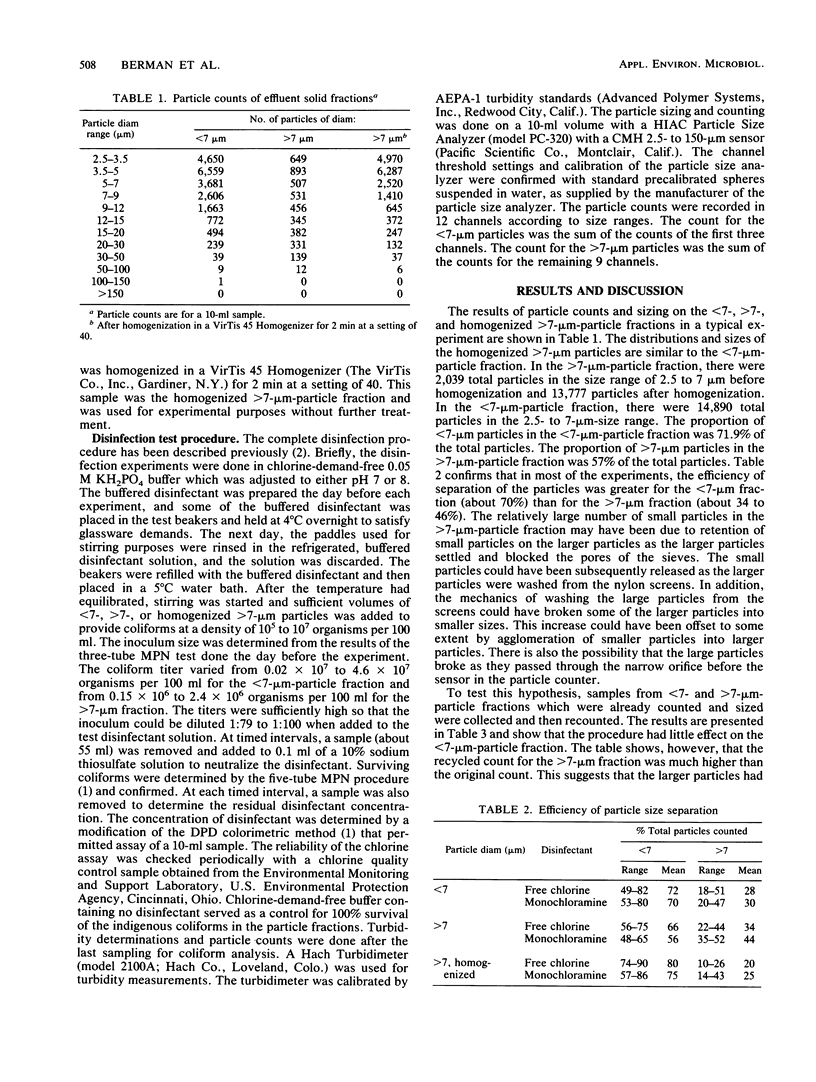
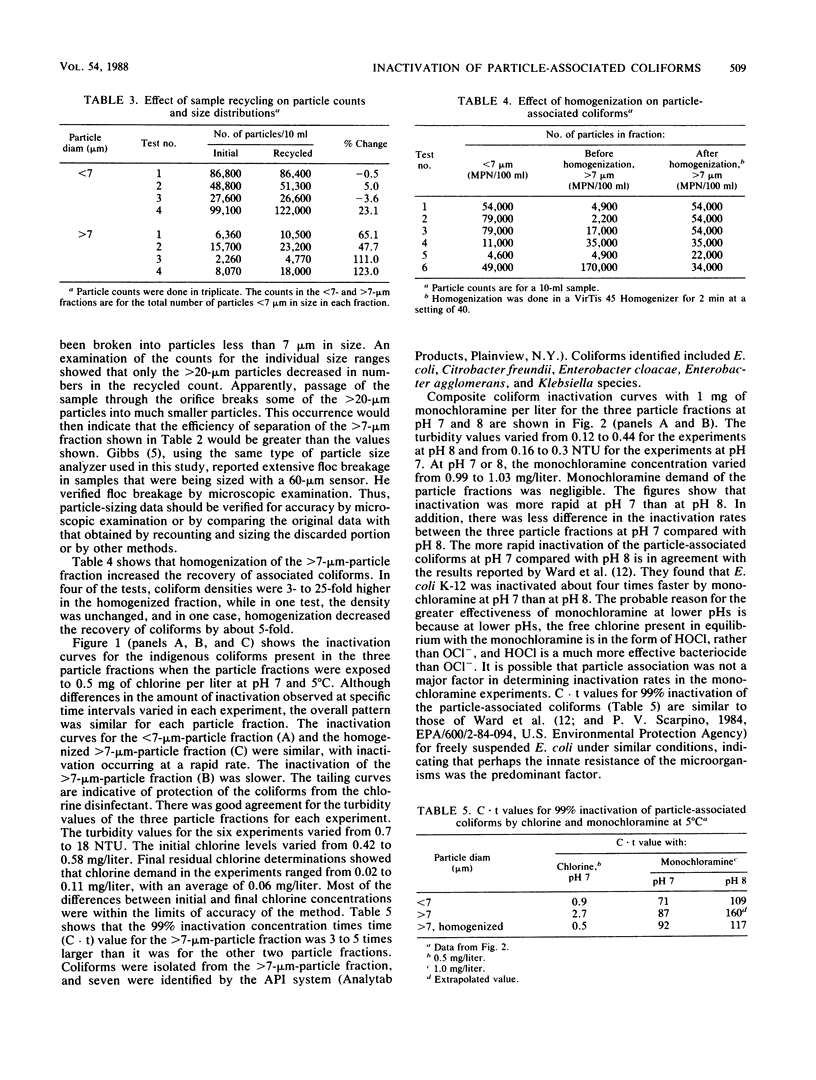
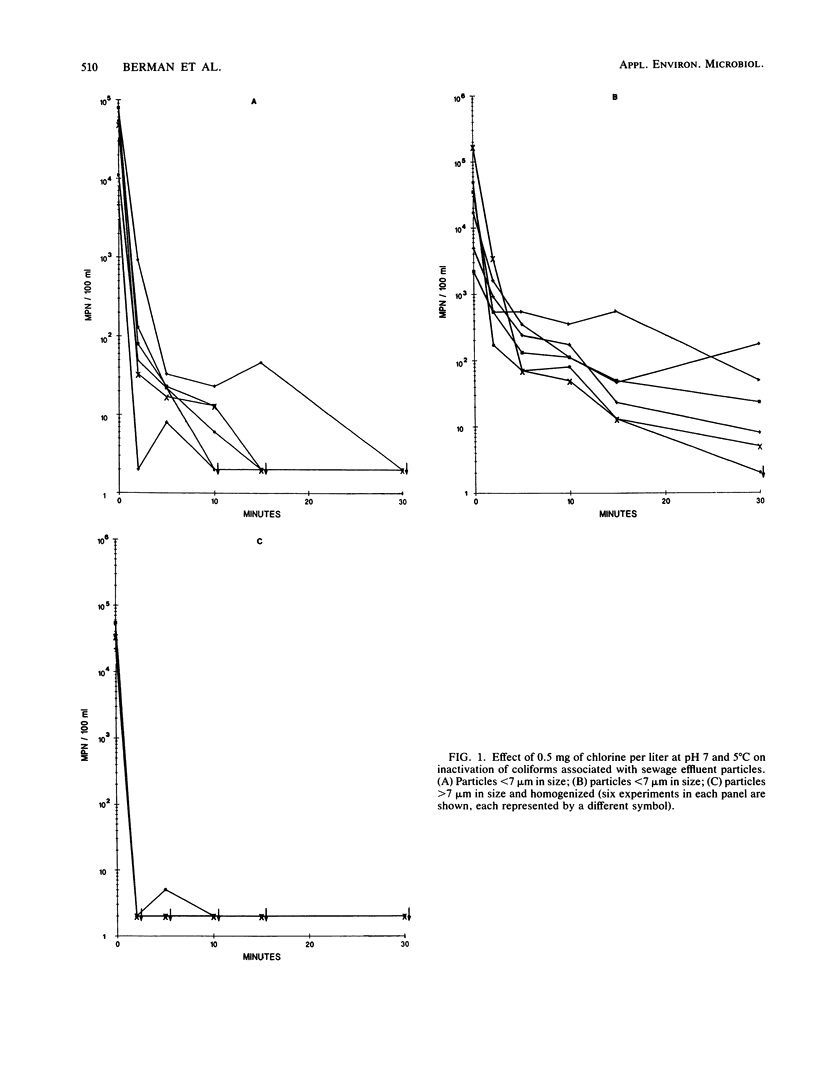
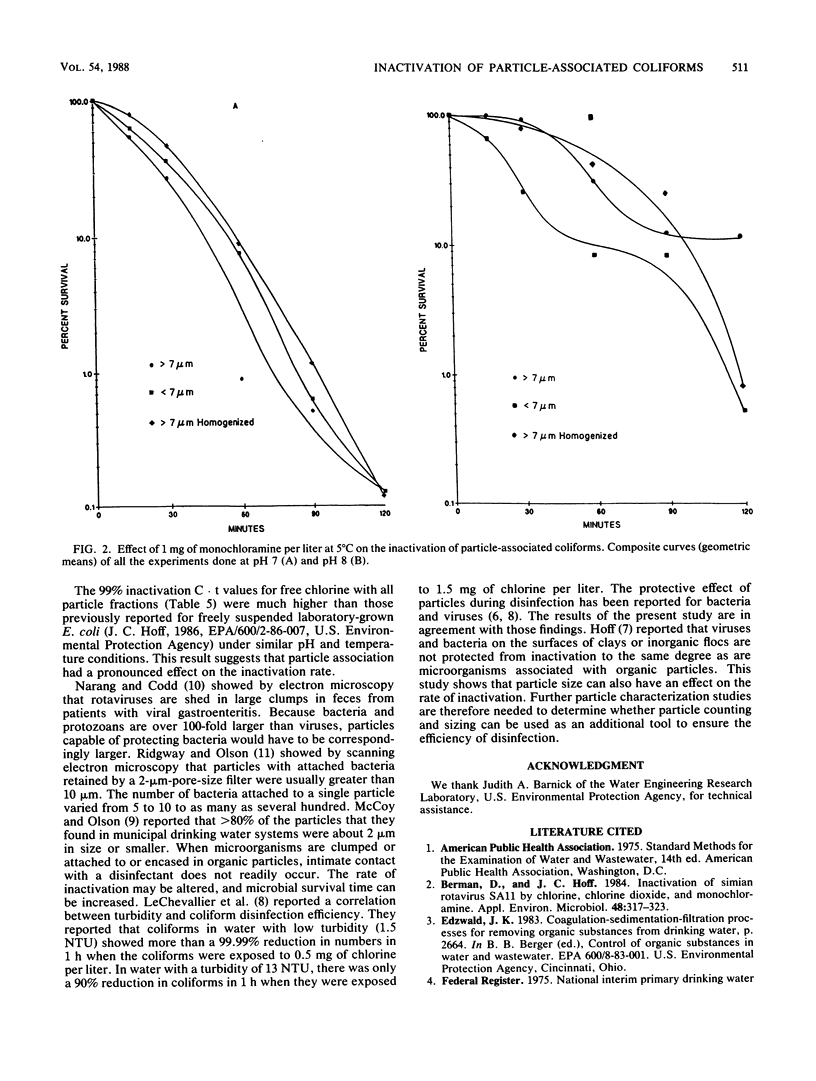
Sieves and nylon screens were used to separate primary sewage effluent solids into particle fractions of less than 7- or greater than 7-micron size. The efficiency of separation was determined by using a particle counter. Indigenous coliforms associated with the particle fractions were tested for their resistance to chlorine and monochloramine. Coliforms associated with the less than 7-microns fraction were inactivated more rapidly by 0.5 mg of chlorine per liter at 5 degrees C and pH 7 than coliforms associated with the greater than 7-microns fraction. Homogenization of the greater than 7-microns fraction not only resulted in an increase in the number of less than 7-microns particles, but also increased the rate of inactivation to a rate similar to that of the less than 7-microns fraction. With 1 mg of monochloramine per liter at 5 degrees C and pH 7, particle size had no appreciable effect on the rate of inactivation. At pH 8, however, the less than 7-micron fraction was inactivated more rapidly than the greater than 7-micron fraction. The time required for 99% inactivation of the particle fractions with monochloramine at pH 7 or 8 was 20- to 50-fold greater than the time required for the same amount of inactivation with chlorine at pH 7. The results indicate that coliforms associated with sewage effluent particles are inactivated more rapidly with 0.5 mg of chlorine per liter than with 1.0 mg of monochloramine per liter. However, greater than 7-micron particles can have a protective effect against the disinfecting action of chlorine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman D., Hoff J. C. Inactivation of simian rotavirus SA11 by chlorine, chlorine dioxide, and monochloramine. Appl Environ Microbiol. 1984 Aug;48(2):317–323. doi: 10.1128/aem.48.2.317-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff J. C., Akin E. W. Microbial resistance to disinfectants: mechanisms and significance. Environ Health Perspect. 1986 Nov;69:7–13. doi: 10.1289/ehp.86697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Evans T. M., Seidler R. J. Effect of turbidity on chlorination efficiency and bacterial persistence in drinking water. Appl Environ Microbiol. 1981 Jul;42(1):159–167. doi: 10.1128/aem.42.1.159-167.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy W. F., Olson B. H. Analysis of the microbiological particulates in municipal drinking-water by scanning electron microscopy/X-ray energy spectroscopy. Zentralbl Bakteriol Mikrobiol Hyg B. 1987 Apr;183(5-6):511–529. [PubMed] [Google Scholar]
- Narang H. K., Codd A. A. Frequency of preclumped virus in routine fecal specimens from patients with acute nonbacterial gastroenteritis. J Clin Microbiol. 1981 May;13(5):982–988. doi: 10.1128/jcm.13.5.982-988.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Olson B. H. Chlorine resistance patterns of bacteria from two drinking water distribution systems. Appl Environ Microbiol. 1982 Oct;44(4):972–987. doi: 10.1128/aem.44.4.972-987.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N. R., Wolfe R. L., Olson B. H. Effect of pH, application technique, and chlorine-to-nitrogen ratio on disinfectant activity of inorganic chloramines with pure culture bacteria. Appl Environ Microbiol. 1984 Sep;48(3):508–514. doi: 10.1128/aem.48.3.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


