Abstract
Dietary flavonoids and other polyphenols show great potential as cancer chemopreventive agents in cell culture studies. This does not translate well into in vivo activity, because of extensive conjugative metabolism of these compounds in the intestine and liver. This paper presents a review of a flavonoid subclass in which all hydroxyl groups are capped by methylation. This results in dramatically increased metabolic stability and membrane transport in the intestine/liver, thus improving oral bioavailability. The methoxyflavones also show increased cancer chemopreventive properties. At the cancer initiation stage, bioactivation of polyaromatic hydrocarbon carcinogens and binding to DNA are markedly diminished through effects on CYP1A1/1B1 transcription but also through direct interactions with the proteins. At the cancer promotion stage, the proliferation of cancer cells, but not normal cells, is inhibited with greater potency than with the unmethylated flavones. Limited mechanistic experiments, such as of effects on cell cycle regulation, indicate that the mechanisms of methoxyflavone activities are unique, including aromatase inhibition. The cancer preventive effects and mechanisms of the polymethoxyflavones, such as tangeretin and nobiletin, are discussed in comparison. It is concluded that the methoxyflavones have properties that may make them particularly useful as cancer chemopreventive agents.
Keywords: Methoxyflavones, Flavonoids, Chemoprevention, Cancer prevention, Bioavailability
1. Introduction
Dietary flavonoids and other polyphenolic food components have over many years been suggested to have preventive properties both at the initiation and the promotion stages of chemically-induced carcinogenesis [1-3]. At the cancer initiation stage, mainly in cell culture studies, polyphenols have clearly been shown to affect many of the carcinogen bioactivating steps necessary for the covalent binding of the carcinogen to cellular DNA, including the major bioactivating CYP1A1 enzyme. Whereas some polyphenols have been shown to act as inducers of CYP1A1 by being agonists of the arylhydrocarbon receptor (AhR), others have been shown to be inhibitors by being AhR antagonists [4-6], although recent studies show that this may be a too simplistic view (see below). At the promotion stage, cell culture studies have revealed a wide variety of biochemical mechanisms for the effects of polyphenols on human cancer cells. This most recently includes effects on VEGF and HIF-1 expression via PI3K/AKT pathways [7] or via ARNT [8], inactivation of EGFR [9] and inhibition of thioredoxin reductase [10], thymidylate synthase [11] and the MDM2 oncogene [12] as well as effects on cancer cell resistance by targeting the molecular chaperone glucose-regulated protein 78 [13].
However, in vivo cancer chemoprevention studies in animals and especially in humans, using modest, clinically tolerable doses of flavonoids or other polyphenols, have been mostly disappointing. This can be explained by the very poor oral bioavailability of the polyphenols, i.e. their inability to pass intact through the dual intestinal/hepatic barrier into the systemic circulation. In humans, this lack of bioavailability after oral doses has been shown directly for chrysin [14], quercetin [14, 15], curcumin [16] and resveratrol [17, 18]. Although the tea flavonoids [19-21] and the isoflavonoids, such as genistein [21-24], have some oral bioavailability, it is still low.
This review will present recent observations on a subclass of flavonoids, i.e., the methoxylated flavones, which may have chemopreventive properties superior to the more common unmethylated flavonoids or polyphenols. The focus will in first hand be on their oral bioavailability and in second hand on their potential cancer chemopreventive properties at both the initiation and the promotion stages of chemically induced carcinogenesis. Effects of the polymethoxyflavones will be discussed for comparison.
2. Lack of Oral Bioavailability of Polyphenols
The ability of nutritional small molecules as well as drugs to produce a biological effect depends in first hand on their ability to enter the target cell. For example, when bronchial epithelial cells were exposed to two dimethoxylated flavones and resveratrol, within five minutes all accumulated 30- to 50-fold in the cells compared to the surrounding buffer [25]. This situation seems to be similar for most flavonoids and other polyphenols. Thus, these compounds easily penetrate all cultured cells, including those from peripheral organs, such as the heart, lungs, breast, prostate, liver, kidneys and brain. Except for hepatic and intestinal cells, cultured cells usually also have low expression of enzymes capable of metabolizing dietary compounds as well as drugs. That includes enzymes such as the cytochrome P450 (CYP) system, the UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs). Thus, for most cell culture studies with dietary polyphenol aglycones, in contrast to their glycosides, there is no limitation in cellular uptake due to poor membrane penetration or possible efflux transporters and/or metabolic instability.
However, when the polyphenols are administered orally to animals or humans, very little or none of these compounds appears in the systemic circulation. The reason is the very high expression of in particular the UGTs and SULTs in the small intestine and liver, through which all of the oral dose must pass. This results in a very low oral bioavailability. Additionally, most dietary flavonoids and other polyphenols are present in the food as glycosides, further decreasing the bioavailability.
3. Metabolic Resistance of Methoxylated Flavones
As clearly demonstrated for the flavonoid chrysin (5,7-dihydroxyflavone), but also for many other polyphenols, glucuronidation and sulfation, and much less so oxidation, are critical for the very rapid metabolism of this compound in the human intestine and liver [26], resulting in essentially zero bioavailability in vivo in humans [14]. However, if the two hydroxyl groups in chrysin are methylated, as in 5,7-dimethoxyflavone (5,7-DMF) (structures, see Fig. 1), the situation is entirely different. Using the human liver 9,000g supernatant (S9 fraction), which contains the microsomal as well as cytosolic enzymes, with the cofactors for glucuronidation (UDPGA), sulfation (PAPS) and oxidation (NADPH), chrysin was rapidly metabolized, with no parent compound remaining after a 20-min incubation. In contrast, 5,7-DMF was metabolically stable over the whole 60-min time-course studied [27, 28]. Quercetin and resveratrol, two common unmethylated dietary polyphenols, as well as many others behaved very similarly to chrysin, i.e., were rapidly metabolized.
Fig. 1.
Structures of 5,7-dihydroxyflavone (chrysin) and 5,7-dimethoxyflavone (5,7-DMF).
In further experiments, the transport of 5,7-DMF compared with chrysin was examined in Caco-2 cells cultured as monolayers on permeable membrane support, a well established human intestinal transport model [29, 30]. The transcellular transport of 5,7-DMF was about 10-fold higher than for chrysin [28]. The reason for this difference is likely not due to differences in transport per se but rather due to differences in metabolic stability, similar to that seen with the hepatic S9 fraction (see Fig. 4). Similarly, 7-methoxyflavone, 7,4′-dimethoxyflavone and 5,7,4′-trimethoxyflavone were much more metabolically stable and had higher transport rate than the corresponding unmethylated flavones [28].
Fig. 4.
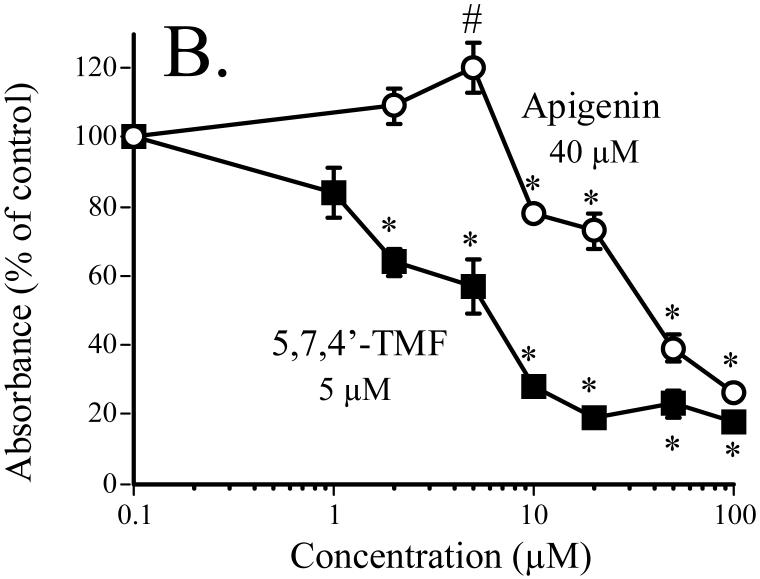
Effect of 5,7,4′-TMF compared to the unmethylated analog apigenin on SCC-9 cell proliferation. Cell proliferation, expressed as percent of control (DMSO-treatment), was measured as BrdU incorporation into cellular DNA after a 24-h exposure of the cells to the flavones [31]. The numbers shown in the figure are the calculated IC50 values. From [31].
* significantly lower than control, P < 0.05. # significantly higher than control, P < 0.05.
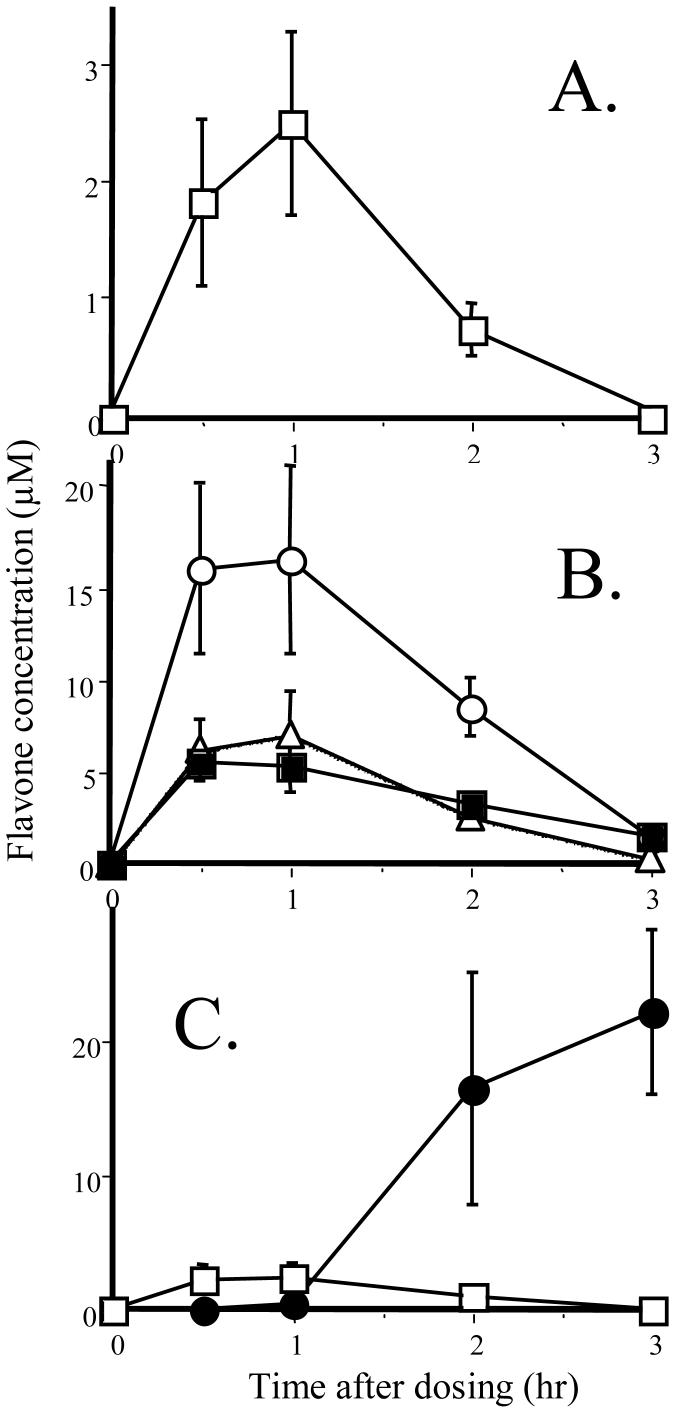
Based on the combined observations with the human hepatic S9 fraction and the human Caco-2 cell system, it could be predicted that the oral bioavailability would be much greater for the methoxyflavones than for the unmethylated flavones. This was tested directly in vivo in the rat, an animal model known to have much greater metabolic capacity than humans, thus, a stringent test [31]. 5,7-DMF and chrysin were co-administered by oral gavage at 5 mg/kg, which is a common dose when chrysin is used as a dietary supplement in humans. Only 5,7-DMF was detectable in the plasma, peaking at 2.3 μM at 1 hr (Fig. 2A). 5,7-DMF was also easily detectable in liver, lung and kidney tissue at quite high concentrations compared to plasma (Fig. 2B). Chrysin was not detected in any tissue but started to appear in the fecal pellets in the intestinal lumen after 2 hr (Fig. 2C). This is likely the result of intestinal absorption, enzymatic conjugation, MRP2-mediated export of the conjugates, enzymatic hydrolysis back to chrysin and fecal excretion, as described previously both in vitro [32] and in vivo in humans [14].
Fig. 2.
Plasma and tissue levels of 5,7-DMF and chrysin after oral administration of 5 mg/kg in rats; (A) Plasma 5,7-DMF (no chrysin could be detected at any time-point); (B) 5,7-DMF in post-absorption tissues, liver (○), lung (■) and kidney (Δ); (C) 5,7-DMF (□) and chrysin (●) in the proximal 2 cm of the colon with the associated fecal pellet. The data represent the mean ± SEM of 5 animals at each time-point. From [31].
The oral bioavailability, based on tissue measurements, was also determined for 5,7-DMF and chrysin in a small fish model, the Atlantic killifish [33]. The fishes (about 4-6 g) were exposed to these flavones in the water at a concentration of 5 μM, a low human dietary concentration, for 8 h prior to sacrifice. The 5,7-DMF concentrations greatly exceeded those of chrysin in all tissues, most notably in the brain. The findings are very similar to those in the rat study. It should be noted that the killifish, being a saltwater species, ingests foreign chemicals by swallowing the compounds, i.e. similarly to oral administration in mammals. The very high 5,7-DMF concentration in the brain (5,7-DMF/chrysin tissue concentration ratio 150) is of potential importance for treating tumors within the CNS, in general a difficult task.
To our knowledge, very few studies exist on the oral bioavailability of other methoxyflavones or polymethoxyflavones. One is the citrus flavonoid nobiletin (5,6,7,8,3′,4′-hexamethoxyflavone), which was administered together with the unmethylated luteolin (5,7, 3′,4′-tetrahydroxyflavone) to rats at 25 mg/kg by intubation [34]. The nobiletin content in the liver and kidneys ranged from 2 to 6 μM, i.e. somewhat less than for 5,7-DMF, but the luteolin levels were much lower, more similar to chrysin. On the other hand, another polymethoxyflavone, the citrus flavonoid tangeretin (5,6,7,8,4′-pentamethoxyflavone), was administered to hamsters as 1% of their diet for 35 days. There was evidence of considerable intestinal absorption of tangeretin based on the urinary excretion of several metabolites in these animals. However, no unchanged tangeretin was detected in the circulating plasma [35].
These data taken together indicate that the methoxylated flavones have a great advantage over the nonmethylated flavones regarding oral bioavailability. It also appears that the compounds containing only one or two methoxy groups may be more metabolically stable than the polymethoxylated flavones, although more studies in this area are needed.
4. Inhibition of Cancer Initiation
It is well recognized that most types of cancers are due, at least in part, to exposure to environmental chemicals, including tobacco smoke, but also to similar contaminants in our diet [36]. The largest class of such chemicals is the polyaromatic hydrocarbons (PAHs) with benzo[a]pyrene (BaP) considered a model compound. The bioactivation of BaP to the form that ultimately binds to DNA to start the carcinogenic process, i.e. cancer initiation, has been well established [36].
It is also clear that BaP potently induces both of the major bioactivating enzymes CYP1A1 and CYP1B1 via activation of the AhR. However, cell context is highly important for determining which of the isoforms is predominantly expressed. Thus, among human cell types after BaP induction, hepatocytes express exclusively CYP1A1 [37], lung epithelial cells mainly CYP1A1 but also some CYP1B1 [25], oral epithelial cells preferentially CYP1B1 [38] and esophageal epithelial cells exclusively CYP1B1 [39].
The effects of flavonoids and other polyphenols on these two bioactivating oxidative enzymes have been studied thoroughly in cell culture models and are highly complex. Some may be AhR agonists, whereas others are antagonists [4-6]. However, it appears that responses very much depend on cell type. With regard to oral bioavailability, most unmethylated polyphenols studied in the past, e.g., quercetin, kaempferol, diosmetin, curcumin and resveratrol, would not be expected to reach internal organs, i.e. beyond sites along the gastrointestinal tract.
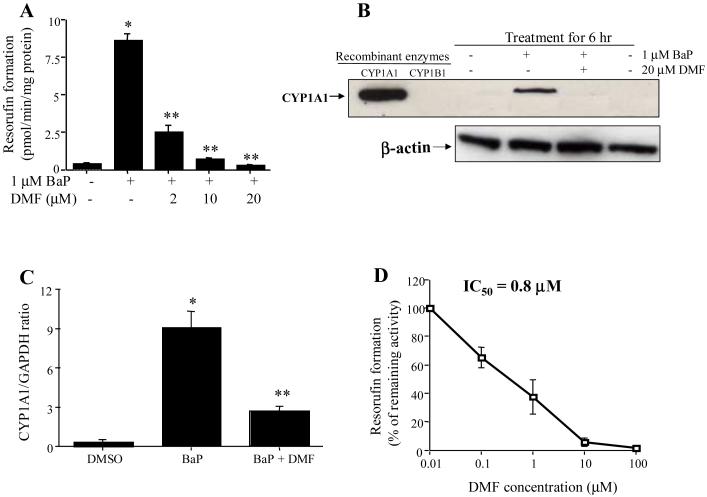
The orally bioavailable methoxyflavones have considerably greater potential to reach target tissues to exert biological effects in vivo. Their potential to inhibit the bioactivation of BaP in vitro has been shown for 3′,4′-DMF [25, 38-40] and 5,7-DMF [25, 37-39] and may also include additional methoxyflavones [41]. 5,7-DMF appears to be highly effective in inhibiting BaP bioactivation and binding to DNA in cell culture. Thus, after exposure of human hepatoma Hep G2 cells to a low concentration of BaP, the BaP-DNA binding was dramatically induced, second to CYP1A induction [37]. 5,7-DMF dramatically reduced the catalytic activity of CYP1A1 (Fig. 3A) and both CYP1A1 protein (Fig. 3B) and mRNA (Fig. 3C) were clearly reduced. In addition, 5,7-DMF was shown to be a highly potent direct inhibitor of recombinant CYP1A1 protein activity (Fig. 3D). Thus, 5,7-DMF has two modes of action on CYP1A1 at quite low (1-2 μM) concentrations. It is important to note that these 5,7-DMF concentrations can easily be reached in tissues, as shown after oral dosing in the rat (Fig. 3B). Thus, studies of the effectiveness of 5,7-DMF treatment in vivo seem to be the next important step.
Fig. 3.
Effects of 5,7-DMF on BaP-induced CYP1A1 activity (A), CYP1A1 protein expression (B) and CYP1A1 mRNA level (C) in the Hep G2 cells and on recombinant CYP1A1 activity (D). A-C: Hep G2 cells were exposed for 6 hr to different concentrations of 5,7-DMF in the presence or absence of 1 μM BaP. D: Recombinant CYP1A1 was incubated with various concentrations of 5,7-DMF [37].
Similarly, both 5,7-DMF and 3′,4′-DMF were potent inhibitors of BaP-induced CYP1A1 protein expression in human bronchial epithelial cells [25]. As shown in Fig. 3B, oral 5,7-DMF administration in the rat resulted in considerable concentrations in the lungs (6 μM), suggesting potential in vivo effectiveness of 5,7-DMF in the chemoprevention also of lung cancer. Thus, studies of 5,7-DMF in animal models are urgently needed.
5,7-DMF appeared to be an inhibitor of CYP1B1 transcription in the human esophageal HET-1A cells [39] but not in the human oral SCC-9 cells [38]. This demonstrates that CYP1B1 regulation is different from that of CYP1A1, at least as far as 5,7-DMF is concerned, but also that cell context is highly important [42, 43]. In contrast, the AhR antagonist 3′,4′-DMF [40] was an inhibitor of CYP1B1 both at the mRNA and protein levels in both esophageal and oral cells. From these observations it is clear that more mechanistic studies of the methoxyflavones are needed.
The mechanism(s) by which the methoxyflavones affect the transcription of CYP1A1/1B1 is complex. 3′,4′-DMF has been shown to be an AhR antagonist in human breast cancer cells, directly binding to the receptor [40]. However, whereas 5,7-DMF apparently inhibits CYP1A1 transcription in Hep G2 [37], SCC-9 [38] and BEAS-2B [25], in neither case was there evidence for an effect on AhR nuclear translocation (unpublished). The mechanism of the 5,7-DMF effect on CYP1A1 transcription thus remains to be elucidated. It may include effects on regulators of AhR, such as hsp90, as shown for the tea flavonoid EGCG [44], or ARNT, as shown for the yellow spice curcumin [8] or maybe the stability of the CYP1A1 mRNA [45].
5. Inhibition of Cancer Promotion
Central to protective effects by dietary flavonoids and other polyphenols at the promotion stage of chemically-induced carcinogenesis is the ability to inhibit cell proliferation. The damage that the carcinogens have inflicted on cellular DNA during the initiation stage is being propagated into a new cell population. This machinery, i.e. clonal expansion, is highly complex, geared towards giving the cells immortality by stimulating mitogenesis and/or decreasing cell death by inhibiting apoptosis. Protective effects at this stage are critically important. This has been demonstrated in cell culture with unmethylated flavonoids and other polyphenols, as discussed briefly in the Introduction, affecting numerous signal transduction pathways.
Some of the polymethoxylated citrus flavonoids have also in preliminary studies demonstrated antiproliferative properties [46, 47] (see Section 6.). However, the effect of methylation of the flavonoids on their antiproliferative effects has not been clarified. This is in particular true for the smaller methoxylated flavones. Many studies in the past have assumed that the free hydroxyl groups of the flavonoids and other polyphenols are necessary for biological effects. The long held belief that their antioxidant properties were responsible for the antiproliferative effects has now been found not to be true [48].
The antiproliferative effects of methoxylated versus hydroxylated flavones were directly compared in SCC-9 human oral squamous carcinoma cells. The effects of treatment with 5,7,4′-trimethoxyflavone (5,7,4′-TMF) versus 5,7,4′-trihydroxyflavone (apigenin), one of the most thoroughly studied unmethylated flavones, are shown in Fig. 4. 5,7,4′-TMF was about 8 times more potent than apigenin with an IC50 value of 5 μM. The IC50 value of 40 μM for apigenin agrees with most previous studies of this flavone in various human cancer cell lines [49-51]. Very similar results as with 5,7,4′-TMF were obtained for 5,7-dimethoxyflavone (5,7-DMF) compared to its unmethylated analog 5,7-dihydroxyflavone (chrysin) [31]. The greater potency of the two methoxylated versus the two hydroxylated flavones could conceivably be due to greater cell uptake of the methoxylated flavones. However, after incubation of SCC-9 cells for up to 24 hr with 25 μM 5,7-DMF or chrysin, the uptake was rapid and virtually identical for the two compounds [31].
A small number of additional methoxylated flavones have been investigated for antiproliferative effects in the SCC-9 cells [31]. The calculated IC50 values were 36.5 μM (7-MF), 24.2 μM (7,4′-DMF), and 19.3 μM (tangeretin). In addition, 5,4′-DMF, 5,3′-DMF and 7,8-DMF showed weaker effects. In MCF-7 human breast cancer cells treated with a small number of hydroxy- and methoxy-substituted flavonoids, the 5-, 7-, and 5,7-methoxyflavanones, i.e., without the C-ring double bond, showed growth inhibitory effects with IC50 values around 35 μM, as measured by the MTT assay [52]. 5-Methoxyflavone was almost as potent as the flavanone and 7-methoxyflavone was more potent than its hydroxyl analog.
To determine whether the growth inhibitory effect was accompanied by cell cycle arrest, SCC-9 cells were treated with two pairs of flavones for 72 h. These experiments showed that apigenin caused a distinct increase in the G2/M phase population, as has previously been shown in several studies [50, 51]. In contrast, 5,7,4′-TMF caused a dose-dependent increase in the G1 phase, significant already at 5 μM, with a concomitant decrease in the S phase and no change in the G2/M population [31]. Identical results were obtained for 5,7-DMF compared to its unmethylated analog chrysin. The results seen by flow cytometry were thus very similar to those seen in the cell proliferation assay.
To determine if the potent antiproliferative effects observed by the methoxylated flavones on the SCC-9 cells were selective for cancer vs. noncancer cells, the effects of 5,7-DMF and chrysin in two additional cancer cell lines were compared with those in two noncancer cell lines. In the FaDu human larynx SCC cells, both 5,7-DMF and chrysin showed similar potency as 5,7-DMF in the SCC-9 cells (IC50 8-10 μM). In the MCF-7 human breast cancer cells, both compounds again had similar but slightly lower potency with IC50 values of 10-20 μM. In contrast, two normal but transformed human cell lines, i.e. the HET-1A esophageal cells [53] and the BEAS-2B bronchial epithelial cells [54], were much less sensitive to both 5,7-DMF and chrysin with IC50 values > 100 μM.
The mechanism of the potent antiproliferative effects of 5,7,4′-TMF and 5,7-DMF compared to their unmethylated analogs has not yet been addressed. However, recent thinking [55, 56] suggests that the effect of these methoxyflavones on the AhR-mediated expression, in particular of CYP1A1, may have a direct effect on cell cycle regulation. This is too early to conclude, but may provide a novel set of mechanisms to pursue, connecting cancer initiation with promotion, including the protective effects of the methoxyflavones.
A specific cancer protective mechanism against hormone-sensitive cancers, which has received much interest lately, is through downregulation of estrogen concentrations. Thus, two methoxylated flavones, 7-methoxyflavone and 7,4′-dimethoxyflavone, have very recently been shown to be potent inhibitors of aromatase, the enzyme responsible for converting testosterone to estradiol (IC50 values of 2-9 μM) [57]. Some flavonoids, notably chrysin, have been shown to be potent aromatase inhibitors in vitro [58]. However, due to lack of bioavailability [14, 27, 28], their claims of therapeutic efficacy have never been substantiated. With the methoxylated flavones, this clinical application may be realistic.
6. Effects of Polymethoxylated Flavones
In SCC-9 oral cancer cells the citrus polymethoxyflavone tangeretin had an antiproliferative IC50 value of about 20 μM [31]. This is lower than in some other studies of this flavone in a colorectal carcinoma cell line (37 μM) [59] as well as in lung and breast carcinoma cell lines (≈ 100 μM) [60] [[61]. A very recent study reported IC50 values of 30-40 μM for breast and colon cell lines with slightly higher values for nobiletin [62]. However, two additional studies of tangeretin in a variety of human cancer cell lines and in promyelocytic leukemia cells reported much more potent effects [47, 63]. These highly variable results may be related to both cell type, length of exposure and methodology for assessing effects.
With the citrus polymethoxylated flavones, as with most flavonoid or polyphenol potential cancer preventive agents, many different mechanisms for their effects have been suggested. G1 cell cycle arrest was induced by tangeretin in human colorectal carcinoma cells [59] and by tangeretin and nobiletin in breast and colon cancer cells [62], just as 5,7,4′-TMF in the oral cells [41]. This was shown to depend on modulation of the activities of several key G1 regulatory proteins, such as Cdk2 and Cdk4, and/or the Cdk inhibitors p21 and p27 [59]. In extended studies, tangeretin was shown to have anti-inflammatory properties in lung epithelial carcinoma cells [60]. Thus, pretreatment of cells with tangeretin inhibited IL-1β-induced p38 MAPK, JNK and AKT phosphorylation and the downstream activation of NF-κB, leading to COX-2 downregulation. Interestingly, while ERK was not affected in this study, a study in estrogen-treated mammary ductal carcinoma cells demonstrated inhibition of ERK phosphorylation by tangeretin [61]. COX-2 inhibition by nobiletin was seen in murine macrophages [64]. The same study showed significant inhibition of dimethylbenz[a]anthracene-induced skin cancer by topically applied nobiletin. Finally, in another recent study, nobiletin inhibited the growth of several prostate cancer cell lines with IC50 values around 100 μM and significantly increased G0/G1 phase arrest. Most interesting, nobiletin administered in the feed for 15 weeks inhibited developing adenocarcinomas of the prostate in a transgenic rat model. This may be the first study indicating in vivo anticancer activity of an orally administered polymethoxyflavone [65].
Several groups have tested nobiletin as an anti-metastatic agent. As overexpression of matrix metalloproteinases (MMPs) is associated with cancer metastasis, this was one target protein. MMP-1 and -9 expression was suppressed by nobiletin in fibrosarcoma cells with associated increase in tissue inhibitors of MMPs [66]. MMP-7 was downregulated in colorectal cells [67] and proMMP-9 activity was inhibited in gastric cell lines [68]. The latter study also showed significantly decreased peritoneal dissemination of stomach cancer nodules, when nobiletin was administered subcutaneously to mice through an osmotic minipump.
7. Where Can the Methoxylated Flavones Be Found?
The polymethoxyflavones, including tangeretin, sinensetin and nobiletin, which have been known for a long time, can be found in high concentrations in the peel of various Citrus species whereas the many hydroxylated flavones [69, 70] clearly predominate in the juice. These polymethoxyflavones contain from 5 to 7 methoxy groups. The various Citrus species show very high variability in their content of these polymethoxyflavones.
The smaller methoxyflavones, with one to three methoxy groups and without any hydroxyl groups, have been much less studied. The reasons are probably two: first, the flavonoids, have been thought to owe their biological activities to the antioxidant properties conferred by free hydroxyl groups. Second, these methoxyflavones are present in plants that are less utilized commercially for human consumption compared to the polymethoxyflavones. For example, 5,7,4′-TMF, although present in a Citrus species [71], also is present in other plants used in folk medicine [72, 73]. 7,4′-DMF has been identified in fruits and leaves from neotropical nutmeg species [74, 75] as well as from propolis [76]. 5,7-DMF is highly abundant in pepper tree leaves [77]. While none of the methoxylated flavones examined in this study are abundant in the common human diet, the mounting evidence of protective properties of these flavones may lead to increased use of their natural sources.
However, a most important aspect of the small methoxylated flavones is their availability in synthetic form. These molecules have apparently been made as building blocks in organic synthesis of potential novel anticancer drugs. Thus, further future studies of methoxyflavones should therefore be greatly facilitated.
8. Future Studies
A very recent epidemiological study provides strong support for the methoxylated flavones as potential cancer chemopreventive dietary agents. This prospective study in 42,311 men in the Health Professionals Follow-up Study [78] convincingly showed that histologically diagnosed oral premalignant lesions were suppressed by consumption of citrus fruits and citrus fruit juices (a 30 to 40% lower risk), whereas, surprisingly, vegetables provided no protection. This is the first epidemiological study providing evidence of protective effects of a subclass of dietary fruits and vegetables. What first comes to mind from these findings is the presence of high concentrations of methoxylated flavones in the essential oils of citrus fruit peel. This includes compounds such as sinensetin, nobiletin, tangeretin and heptamethoxyflavone [69, 70] but most likely many others, including the simpler methoxyflavones similar to those described in this chapter. This should provide a particular incentive for further studies of these dietary chemicals.
There are two general approaches to the use of flavonoids or other polyphenols as cancer chemopreventive agents. One is to choose diets rich in such compounds, including verification of their possible effectiveness followed by attempts to deduce potential mechanisms. Examples of this whole food approach include herbal teas [21], soy incorporation in the diet [24] as well as a berry approach [79]. The other more common and simplest approach is to identify dietary components with potential cancer chemopreventive properties through testing of individual compounds first in vitro and then in vivo. The ultimate goal here is to arrive at food supplements, which may serve to reinforce the dietary contribution. The new methoxyflavones may fit into either or both of these categories.
Acknowledgements
T.W. is supported in part by the National Institutes of Health Grant GM55561. The help of Kristina Walle in the preparation of this manuscript is greatly acknowledged.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- BaP
benzo[a]pyrene
- Cdk
cyclin-dependent kinase
- COX-2
cyclooxygenase-2
- CYP
cytochrome P450
- DMF
dimethoxyflavone
- EGFR
epidermal growth factor receptor
- EGCG
epigallocatechin gallate
- HIF-1
hypoxia-inducible factor 1
- hsp90
heat shock protein 90
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MDM2
murine double minute-2
- MF
methoxyflavone
- MMP
metalloproteinase
- MRP
multidrug resistance-associated protein
- NF-κB
nuclear factor-κB
- PAH
polyaromatic hydrocarbon
- PAPS
3′-phosphoadenosine-5′-phosphosulfate
- PI3K/AKT
phosphatidylinositol 3-kinase/AKT
- S9
9 000 g supernatant
- SULT
sulfotransferase
- TMF
trimethoxyflavone
- UDPGA
uridine 5′-diphosphoglucuronic acid
- UGT
UDP-glucuronosyltransferase
- VEGF
vascular endothelial growth factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Middleton EJ, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- [2].Yang CS, Prabhu S, Landau J. Prevention of carcinogenesis by tea polyphenols. Drug Metab Rev. 2001;33:237–53. doi: 10.1081/dmr-120000651. [DOI] [PubMed] [Google Scholar]
- [3].Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–85. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- [4].Ciolino HP, Daschner PJ, Wang TTY, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- [5].Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340:715–22. [PMC free article] [PubMed] [Google Scholar]
- [6].Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999;56:760–7. [PubMed] [Google Scholar]
- [7].Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang B-H. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–53. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- [8].Choi H, Chun Y-S, Kim S-W, Kim M-S, Park J-W. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–71. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- [9].Hou Z, Sang S, You H, Lee M-J, Hong J, Chin K-V, et al. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–56. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- [10].Lu J, Papp LV, Fang J, Rodriguez-Nieto S, Zhivotovsky B, Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res. 2006;66:4410–8. doi: 10.1158/0008-5472.CAN-05-3310. [DOI] [PubMed] [Google Scholar]
- [11].Haghiac M, Walle T. Quercetin induces necrosis and apoptosis in the SCC-9 oral cancer cells. Nutr Cancer. 2005;53:220–31. doi: 10.1207/s15327914nc5302_11. [DOI] [PubMed] [Google Scholar]
- [12].Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–96. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- [13].Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma W-Y, et al. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–8. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- [14].Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51:143–6. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Rad Res. 2004;38:771–85. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- [16].Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- [18].Walle T, Hsieh F, DeLegge MH, Oatis JEJ, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- [19].Chow HHS, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenol E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- [20].Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131:1731–7. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- [21].Lambert JD, Hong J, Yang G, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81(suppl):284S–91S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- [22].Izumi T, Piskula MK, Osawa S. al. e. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans J Nutr 20001301695–9. [DOI] [PubMed] [Google Scholar]
- [23].Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–62. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- [24].Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–6. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- [25].Tsuji PA, Walle T. Inhibition of benzo[a]pyrene-activating enzymes and DNA-binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis. 2006;27:1579–85. doi: 10.1093/carcin/bgi358. [DOI] [PubMed] [Google Scholar]
- [26].Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30:576–81. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- [27].Wen X, Walle T. Methylation protects dietary flavonoids from rapid hepatic metabolism. Xenobiotica. 2006;36:387–97. doi: 10.1080/00498250600630636. [DOI] [PubMed] [Google Scholar]
- [28].Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34:1786–92. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- [29].Artursson P. Cell cultures as models for drug absorption across the intestinal mucosa. Crit Rev Ther Drug Carr Syst. 1991;8:305–30. [PubMed] [Google Scholar]
- [30].Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175:880–5. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- [31].Walle T, Ta N, Kawamori T, Wen X, Tsuji PA, Walle UK. Cancer chemopreventive properties of orally bioavailable flavonoids - methylated versus unmethylated flavones. Biochem Pharmacol. 2007;73:1288–96. doi: 10.1016/j.bcp.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Walle UK, Galijatovic A, Walle T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol. 1999;58:431–8. doi: 10.1016/s0006-2952(99)00133-1. [DOI] [PubMed] [Google Scholar]
- [33].Tsuji PA, Winn RN, Walle T. Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. Chem-Biol Interact. 2006;164:85–92. doi: 10.1016/j.cbi.2006.08.023. [DOI] [PubMed] [Google Scholar]
- [34].Murakami A, Koshimizu K, Ohigashhi H, Kuwahara S, Kuki W, Takahashi Y, et al. Characteristic rat tissue accumulation of nobiletin, a chemopreventive polymethoxyflavonoid, in comparison with luteolin. BioFactors. 2002;16:73–82. doi: 10.1002/biof.5520160303. [DOI] [PubMed] [Google Scholar]
- [35].Kurowska EM, Manthey JA. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J Agric Food Chem. 2004;52:2879–86. doi: 10.1021/jf035354z. [DOI] [PubMed] [Google Scholar]
- [36].Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–9. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- [37].Wen X, Walle UK, Walle T. 5,7-Dimethoxyflavone down-regulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26:803–9. doi: 10.1093/carcin/bgi015. [DOI] [PubMed] [Google Scholar]
- [38].Wen X, Walle T. Preferential induction of CYP1B1 by benzo[a]pyrene in human oral epithelial cells: Impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis. 2005;26:1774–81. doi: 10.1093/carcin/bgi127. [DOI] [PubMed] [Google Scholar]
- [39].Wen X, Walle T. Cytochrome P450 1B1, a biomarker and chemopreventive target for benzo[a]pyrene-initiated human esophageal cancer. Cancer Lett. 2006;246:109–14. doi: 10.1016/j.canlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [40].Lee J-E, Safe S. 3′,4′-Dimethoxyflavone as an aryl hydrocarbon receptor antagonist in human breast cancer cells. Toxicol Sci. 2000;58:235–42. doi: 10.1093/toxsci/58.2.235. [DOI] [PubMed] [Google Scholar]
- [41].Walle T, Walle UK.Novel methoxylated flavone inhibitors of cytochrome P450 1B1 in SCC-9 human oral cancer cells J Pharm Pharmacol 2007. in press [DOI] [PubMed] [Google Scholar]
- [42].Zhang S, Qin C, Safe S. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–82. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Harper PA, Riddick DS, Okey AB. Regulating the regulator: Factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol. 2006;72:267–79. doi: 10.1016/j.bcp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [44].Palermo CM, Westlake CA, Gasiewicz TA. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44:5041–52. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- [45].Lee J-E, Safe S. Involvement of a post-transcriptional mechanism in the inhibition of CYP1A1 expression by resveratrol in breast cancer cells. Biochem Pharmacol. 2001;62:1113–24. doi: 10.1016/s0006-2952(01)00763-8. [DOI] [PubMed] [Google Scholar]
- [46].Manthey JA, Guthrie N, Grohmann K. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001;8:135–53. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- [47].Manthey JA, Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J Agric Food Chem. 2002;50:5837–43. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- [48].Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: antioxidants and signalling molecules? Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- [49].Chiang L-C, Ng LT, Lin I-C, Kuo P-L, Lin C-C. Anti-proliferative effect of apigenin and its apoptotic induction in Hep G2 cells. Cancer Lett. 2006;237:207–14. doi: 10.1016/j.canlet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [50].Trochon V, Blot E, Cymbalista F, Engelmann C, Tang RP, Thomaidis A, et al. Apigenin inhibits endothelial-cell proliferation in G(2)/M phase whereas it stimulates smooth-muscle cells by inhibiting P21 and P27 expression. Int J Cancer. 2000;85:691–6. doi: 10.1002/(sici)1097-0215(20000301)85:5<691::aid-ijc15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [51].Ujiki MB, Ding X-Z, Salabat MR, Bentrem DJ, Golkar L, Milam B, et al. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006;5:1–8. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pouget C, Lauthier F, Simon A, Fagnere C, Basly J-P, Delage C, et al. Flavonoids: structural requirements for antiproliferative activity on breast cancer cells. Bioorg Med Chem Lett. 2001;11:3095–7. doi: 10.1016/s0960-894x(01)00617-5. [DOI] [PubMed] [Google Scholar]
- [53].Stoner G, Kaighn M, Reddel R, Resau J, Bowman D, Naito Z, et al. Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 1991;51:365–71. [PubMed] [Google Scholar]
- [54].Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–9. [PubMed] [Google Scholar]
- [55].Levine-Fridman A, Chen L, Elferink CJ. Cytochrome P4501A1 promotes G1 phase cell cycle progression by controlling aryl hydrocarbon receptor activity. Mol Pharmacol. 2004;65:461–9. doi: 10.1124/mol.65.2.461. [DOI] [PubMed] [Google Scholar]
- [56].Bock KW, Köhle C. Ah receptor- and TCDD-mediated liver tumor promotion: clonal selection and expansion of cells evading growth arrest and apoptosis. Biochem Pharmacol. 2005;69:1403–8. doi: 10.1016/j.bcp.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [57].Ta N, Walle T.Aromatase inhibition by bioavailable methylated flavones J Steroid Biochem Mol Biol 2007. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kao Y-C, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ Health Perspect. 1998;106:85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pan M-H, Chen W-J, Lin-Shiau S-Y, Ho C-T, Line J-K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–84. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- [60].Chen K-H, Weng M-S, Lin J-K. Tangeretin suppresses IL-1ß-induced cyclooxygenase (COX)-2 expression through inhibition of p38 MAPK, JNK, and AKT activation in human lung carcinoma cells. Biochem Pharmacol. 2007;73:215–27. doi: 10.1016/j.bcp.2006.09.018. [DOI] [PubMed] [Google Scholar]
- [61].Van Slambrouck S, Parmar VS, Sharma SK, De Bondt B, Foré F, Coopman P, et al. Tangeretin inhibits extracellular-signal-regulated kinase (ERK) phosphorylation. FEBS Lett. 2005;579:1665–9. doi: 10.1016/j.febslet.2004.10.114. [DOI] [PubMed] [Google Scholar]
- [62].Morley KL, Ferguson PJ, Koropatnick J.Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells Cancer Lett 2007. in press [DOI] [PubMed] [Google Scholar]
- [63].Hirano T, Abe K, Gotoh M, Oka K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br J Cancer. 1995;72:1380–8. doi: 10.1038/bjc.1995.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000;60:5059–66. [PubMed] [Google Scholar]
- [65].Tang M, Ogawa K, Asamoto M, Hokaiwado N, Seeni A, Suzuki S, et al. Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells Cancer Sci 2007. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, et al. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002;62:1025–9. [PubMed] [Google Scholar]
- [67].Kawabata K, Murakami A, Ohigashi H. Nobiletin, a citrus flavonoid, down-regulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci Biotechnol Biochem. 2005;69:307–14. doi: 10.1271/bbb.69.307. [DOI] [PubMed] [Google Scholar]
- [68].Minagawa A, Otani Y, Kubota T, Wada N, Furukawa T, Kumai K, et al. The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn J Cancer Res. 2001;92:1322–8. doi: 10.1111/j.1349-7006.2001.tb02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lu Y, Zhang C, Bucheli P, Wei D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant Foods Hum Nutr. 2006;61:57–65. doi: 10.1007/s11130-006-0014-8. [DOI] [PubMed] [Google Scholar]
- [70].Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem. 2006;70:178–92. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- [71].Mizuno M, Iinuma M, Ohara M, Tanaka T, Iwamasa M. Chemotaxonomy of the genus Citrus based on polymethoxyflavones. Chem Pharm Bull. 1991;39:945–9. [Google Scholar]
- [72].Jaipetch T, Reutrakul V, Tuntiwachwuttikul P, Santisuk T. Flavonoids in the black rhizomes of Boesenbergia pandurata. Phytochemistry. 1983;22:625–6. [Google Scholar]
- [73].Yenjai C, Prasanphen K, Daodee S, Wongpanich V, Kittakoop P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia. 2004;75:89–92. doi: 10.1016/j.fitote.2003.08.017. [DOI] [PubMed] [Google Scholar]
- [74].Cavalcante SH, Fernandes D, Paulino Fo HF, Yoshida M, Gottlieb OR. Lignoids from the fruit of three Virola species. Phytochemistry. 1985;24:1865–6. [Google Scholar]
- [75].Santos LS, Corréa MJC, Campos LMO, Andrade MA. Constituents from the leaves of Virola michelli. Fitoterapia. 1996;67:555–6. [Google Scholar]
- [76].Popravko SA, Gurevich AI, Kolosov MN. Flavonoid components of propolis. Khimiya Prirodnykh Soedinenii. 1969;5:476–82. [Google Scholar]
- [77].Ahmad F, Bakar SA, Ibrahim AZ, Read RW. Constituents of the leaves of Piper caninum. Planta Med. 1997;63:193–4. doi: 10.1055/s-2006-957648. [DOI] [PubMed] [Google Scholar]
- [78].Maserejian NN, Giovannucci E, Rosner B, Zavras A, Joshipura K. Prospective study of fruits and vegetables and risk of oral premalignant lesions in men. Am J Epidemiol. 2006;164:556–66. doi: 10.1093/aje/kwj233. [DOI] [PubMed] [Google Scholar]
- [79].Stoner G. Introduction. Nutr Cancer. 2006;54:1–2. [Google Scholar]