Abstract
Reperfusion injury, which occurs upon the reintroduction of blood flow to an ischemic organ, is responsible for considerable damage in heart attacks and strokes. However, no treatment for reperfusion injury is currently available. A major cause of reperfusion injury is the iron-mediated generation of hydroxyl radical (⋅OH). In this study we have explored the capacity of novel iron chelators called “exochelins” to prevent reperfusion injury. Exochelins, siderophores of Mycobacterium tuberculosis, are unique iron chelators because they are lipid soluble, and hence able to enter cells rapidly. In the iron-free state, exochelins prevented ⋅OH formation. Desferri-exochelins prevented oxidative injury to cultured cardiac myocytes, and did so more rapidly and effectively than the nonlipid soluble iron chelator deferoxamine. The capacity of various desferri-exochelins to protect myocytes from oxidative injury varied directly with their solubility in lipid. Infused into isolated rabbit hearts during reperfusion after a period of ischemia, desferri-exochelins dramatically improved systolic and diastolic left ventricular function, preserved coronary flow, reduced release of the cardiac enzyme lactic dehydrogenase, and reduced myocardial concentrations of ⋅OH metabolites. Thus, highly diffusible desferri-exochelins block injury caused by ⋅OH production and have potential for the treatment of reperfusion injury.
Reperfusion injury occurs in tissues that have been temporarily deprived of blood flow, as occurs in heart attack, stroke, and cardiopulmonary bypass and organs removed for transplantation. Paradoxically, although re-establishment of blood flow to ischemic tissue is necessary for its survival, reperfusion initiates a second phase of injury. In animal models of ischemia and reperfusion, up to 60% of the total damage to the heart (1) and 73% of the damage to the brain (2) is caused by processes initiated during reperfusion. In recent years, advances have been made in the treatment of the ischemic phase of heart attacks and strokes, especially the use of thrombolytic drugs or angioplasty to open acutely obstructed arteries. However, no treatment has been developed for reperfusion injury.
A well-established mechanism of reperfusion injury is the generation of reactive oxygen species (1, 3, 4). Although hydrogen peroxide (H2O2) and superoxide radical (⋅O2−), are generated during ischemia and reperfusion (3, 4), these species are weak oxidants that are readily neutralized by endogenous scavenger enzymes (5). However, H2O2 and ⋅O2− can participate in iron-catalyzed reactions that generate hydroxyl radical (⋅OH), a highly reactive molecule for which there are no effective endogenous defenses (6–8). The availability of redox-active iron at critical intracellular sites appears to be essential to the cellular damage caused by ⋅OH in reoxygenated tissues, whereas extracellular iron plays little or no role (9). In the cell membrane, the probable site at which oxidant injury to the myocardium occurs, ⋅OH can initiate lipid peroxidation (10) or oxidation of protein sulfhydryl groups (11, 12).
Reperfusion injury begins immediately after reintroduction of blood flow to ischemic tissues. Effective treatment requires a pharmacologic agent that can rapidly access the target tissue and efficiently interrupt the cascade of molecular events that leads to irreversible cell injury. Given the pivotal role of iron in the molecular cascade leading to reperfusion injury, iron chelators are of obvious interest as therapeutic agents for this syndrome. The clinical iron chelator deferoxamine prevents ⋅OH formation (7, 13). However, this lipid-insoluble agent enters cells very slowly by pinocytosis and cannot be given in high doses without adverse hemodynamic effects (14, 15). To reduce myocardial infarct size during ischemia and reperfusion, deferoxamine must be given before ischemia begins (16, 17). Because heart attacks and strokes are unpredictable, the inability of deferoxamine to rapidly enter cells limits its usefulness for treatment of reperfusion injury.
Exochelins are siderophores secreted by Mycobacterium tuberculosis that have been isolated and purified by chloroform extraction and reverse-phase HPLC (18, 19). Exochelins are unique in that they are both lipid and water soluble—characteristics that allow them to diffuse into and through lipid membranes, such as the cell wall of the M. tuberculosis bacillus, and to bind iron in the aqueous milieu of the host in which the bacillus resides and multiplies. We hypothesized that, because of their high solubility in lipid, desferri-exochelins could enter myocardial cells rapidly enough to prevent injury from reactive oxygen species even if they were administered during reperfusion, when lipid-insoluble iron chelators, such as deferoxamine, are ineffective.
MATERIALS AND METHODS
Isolation and Purification of Exochelins.
The highly virulent Erdman strain of M. tuberculosis was cultured for 6 weeks in modified, iron-deficient Sauton’s broth medium (18, 19). Exochelins were isolated and purified from the culture filtrate by chloroform extraction and reverse-phase HPLC (18). The family of exochelins was eluted from a C-18 reverse-phase HPLC column in an acetonitrile gradient. The order of their elution reflects the polarity of each exochelin with the most polar exochelins eluting earliest and the most nonpolar, or most lipid soluble, latest. The individual exochelins were purified on an alkyl phenyl column, converted to the desferri-form by incubation with a high concentration of EDTA and repurified (18).
Adult Rat Cardiac Myocyte Isolation and Culture.
Briefly, male adult rats were anesthetized and the heart chilled in situ by flooding the thoracic cavity with iced Ca2+-free modified Krebs–Ringer bicarbonate buffer (MKRB). The heart was excised, placed on a modified Langendorff apparatus, and perfused with MKRB gassed with 95% O2 and 5% CO2 at 37°C. After an initial single-pass perfusion with Ca2+-free MKRB, the system was switched to a recirculating perfusion with collagenase/hyaluronidase in 50 μM Ca2+-MKRB. The ventricles were separated, coarsely minced, and incubated in a collagenase/trypsin solution at 37°C with 95% O2 and 5% CO2. The tissue-cell suspension then was triturated and filtered into cold trypsin inhibitor solution. The suspension was centrifuged and the pellet was resuspended in a 200 μM Ca2+ solution. The Ca2+ concentration was gradually increased to 1.4 mM, and damaged cells were removed with three 15-min gravity sedimentations. The cell suspension then was plated in laminin-coated plastic dishes in medium containing 1.4 mM CaCl2, MEM with Earle’s salts, amino acids, vitamins, pyruvate, carnitine, creatine, taurine, glucose, insulin, antibiotics, and 5% fetal bovine serum. The final culture medium was the same except that glucose and insulin levels were reduced. The plated cells formed a subconfluent monolayer. Viability was greater than 90% after 24 hr in culture and greater than 80% after 72 hr in culture, as measured by trypan blue exclusion.
Assay for Oxidant-Induced Injury to Cardiac Myocytes.
Lactic dehydrogenase (LDH) release from myocytes or isolated hearts was analyzed spectrophotometrically. Fluorescence was measured with an exciting wavelength of 340 nm and an emitting wavelength of 465 nm for 3 min at room temperature. The “cell injury index,” expressed as a percentage, was equal to the LDH activity in the medium from wells containing cells exposed to oxidant stress divided by the total cellular LDH activity (calculated from LDH in wells in which all cells were lysed with Triton X-100) × 100 (20).
Measurement of Left Ventricular Function in Isolated Rabbit Hearts.
The hearts of pentobarbital-anesthetized, adult New Zealand White rabbits were rapidly excised and perfused by a nonrecirculating Langendorff technique. The perfusate was Krebs–Henseleit buffer containing 1 μM sodium salicylate. A fluid-filled latex balloon was inserted into the left ventricle across the mitral valve. The left ventricle was vented. Buffer perfusate was delivered to the heart through an aortic cannula at a pressure of 73 mm Hg, and desferri-exochelins were infused through a side port. Intraventricular pressure and its first derivative were continuously measured through the balloon. The ventricle was paced by right ventricular electrodes. After a 30-min equilibration period, the aortic cannula was clamped to produce a stop-flow state during which pacing was stopped and ventricular fibrillation ensued. After a 30-min hypoxic period, reperfusion with pacing was reinitiated. During the 30-min reperfusion period, pressures were recorded continuously and coronary effluent was collected every 5 min. At the end of the reperfusion period, the hearts were immediately freeze-clamped with aluminum tongs cooled in liquid nitrogen and stored at −70°C for subsequent analysis.
Cell-Free ⋅OH Generating System.
To create a ⋅OH generating system, we mixed 200 μM xanthine sodium (Sigma), 4.4 milliunits/ml purified xanthine oxidase (kindly provided by Joseph McCord, Webb Waring Institute, Denver, CO), and 1 μM ferric nitrilotriacetate (FeNTA) in water from which iron was removed by Chelex 100 resin (Bio-Rad). The FeNTA was prepared from nitrilotriacetic acid, disodium salt (Sigma), and ferric chloride, hexahydrate (Sigma). In addition to exochelins, we tested the effect of iron chelation with deferoxamine mesylate (CIBA Pharmaceutical).
Salicylate Technique for ⋅OH Radical Detection.
The specific and rapid reaction of hydroxyl radicals with various biochemical compounds containing aromatic rings leads to stable compounds that can be isolated and quantitated. Salicylate has been used as one of these trapping agents (21). The reaction of salicylate with ⋅OH produces two different products depending on whether attack occurs at the 3 or 5 position of the aromatic ring. Other P450 systems also hydroxylate salicylate, primarily or exclusively at the 5 position, and a reliable quantitative analysis requires the independent determination of both salicylate isomers (21).
Myocardium (500 mg) was homogenized in distilled iron-free water (5 ml) and then acidified with 12.1 M hydrochloric acid (250 μl). As an internal standard, 2,6-dihydroxybenzoic acid (DHBA) (500 pmol) was added. Ethyl acetate (2 × 4 ml) was used to extract the myocardial homogenate. Bis-(trimethylsilyl) trifluoroacetamide (50 μl) was added to the dried extracts and standards, and trimethylsilyl ethers were derivatized at room temperature for 60 min. One-microliter aliquots were analyzed by GC/MS.
Data were acquired by a Hewlett Packard 5890 gas chromatograph interfaced to a 5970 mass selective detector and processed by an HP 9000 series data system. Injection port and transfer line temperatures were 225°C and 200°C, respectively. The GC program had an initial column temperature of 100°C for 5 min, then increased to 300°C at a rate of 10°C per min. The ion chromatogram of authentic 2,3-, 2,5-, and 2,6-DHBA demonstrated distinct peaks for each of these isomers. Their spectra was dominated by an intense ion at m/z 355. The ion monitored for the dihydroxybenzoic acids was m/z 355, which is the loss of 15 amu from the molecular ion m/z 370. The ion ratios of 356/355 and 357/355 were used as further assessment of specificity at each retention time by comparison to authentic standards. These ratios then were used to continually assess the authenticity of the GC/MS signals derived from the biological extracts. Confirmation of the biological 2,3- and 2,5-DHBA was achieved by analyzing an aliquot of the extracted homogenate derivative by using GC/MS operated in the scan mode. Comparison of their GC retention times and mass spectra with their corresponding authentic standards demonstrated them to be identical.
Quantitation was achieved by operating the mass spectrometer in the selective ion monitoring mode. The dwell time was 25 ms. A highly linear standard curve for each analyte was obtained over a range of concentrations from 0 to 750 pmol while 500 pmol internal standard was simultaneously measured. The ion ratios of analyte/internal standard, (m/z 355/355) of the standard curves were plotted against their concentrations. The threshold for detection of 2,3- and 2,5-DHBA approached 0.25 pmol (signal/noise = 10:1). In three untreated hearts, we measured 2,3- and 2,5-DHBA in coronary effluent collected throughout reperfusion and in the myocardium after 30 min of reperfusion. We found that 78% of the 2,3-DHBA remained in the myocardium during this period, and that concentrations in the effluent were low and more difficult to measure. Because this isomer of salicylate is highly specific for ⋅OH, and because of the relatively high levels in myocardium, we elected to examine myocardial concentrations to assess ⋅OH production. Concentrations were expressed in pmol/g of myocardium.
Statistical Analysis.
All data are presented as mean ± standard error. Statistical analyses were by ANOVA or linear regression. A P < 0.05 was considered to be significant.
RESULTS
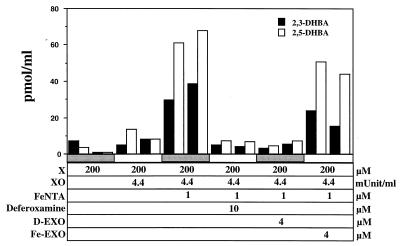
We first investigated whether desferri-exochelins prevent ⋅OH production by studying their effects in a cell-free ⋅OH generating system consisting of xanthine and xanthine oxidase, which produce H2O2 and ⋅O2−, and iron, added as FeNTA. Salicylate was added to trap ⋅OH through hydroxylation (Fig. 1). Without treatment with iron chelators, or when an iron-loaded exochelin was added, ⋅OH was abundantly produced, as evidenced by the accumulation of the hydroxylated salicylate isomers 2,3- and 2,5-DHBA. However, with addition of deferoxamine or a desferri-exochelin, only trace amounts of the hydroxylated salicylate isomers were produced. Therefore, desferri-exochelins, like deferoxamine, prevent ⋅OH production through iron chelation.
Figure 1.
Inhibition of ⋅OH generation by desferri-exochelins. Production of ⋅OH was quantitated by measurements of the hydroxylated salicylate isomers 2,3-DHBA and 2,5-DHBA by GC/MS. A ⋅OH generating system was created by adding 200 μM of salicylic acid to 200 μM xanthine sodium (X), 4.4 milliunits/ml of purified xanthine oxidase (XO), and iron supplied as 1 μM FeNTA. D-EXO is desferri-exochelin 772SM, and Fe-EXO is iron-saturated exochelin 772SM. Duplicate measurements are shown. When X, XO, and FeNTA were present, 2,3- and 2,5-DHBA were produced in the absence of iron chelators or in the presence of Fe-EXO. However, in the presence of 10 μM deferoxamine mesylate or 4 μM D-EXO, production of 2,3- and 2,5-DHBA was suppressed (by preplanned ANOVA, P < 0.01 for 2,3-DHBA and P < 0.001 for 2,5-DHBA). Therefore, both deferoxamine and a desferri-exochelin prevented generation of ⋅OH by iron chelation in this cell-free system.
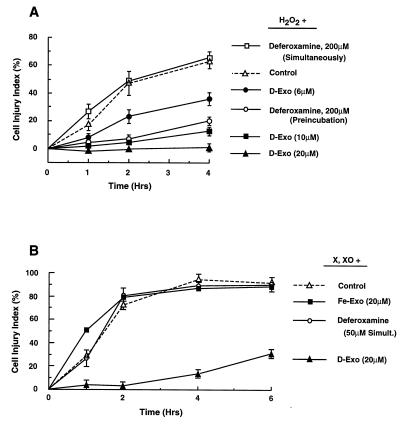
We next examined the protective effects of desferri-exochelins against cytotoxicity in adult rat cardiac myocytes exposed to H2O2 and ⋅O2− in iron-containing culture medium (Fig. 2A). When added simultaneously with H2O2, desferri-exochelin inhibited cytotoxicity, measured by LDH release, in a concentration-dependent manner. Deferoxamine was effective only when preincubated with the myocytes for 2 hr before addition of H2O2. Desferri-exochelins substantially reduced cell injury even when added 20 min after the onset of exposure to H2O2 (data not shown). In myocytes exposed to xanthine and xanthine oxidase to generate both H2O2 and ⋅O2− (Fig. 2B), simultaneous treatment with desferri-exochelins markedly reduced cell injury, but deferoxamine and iron-loaded exochelin were ineffective.
Figure 2.
Desferri-exochelins protect cultured adult rat cardiac myocytes against injury from exposure to reactive oxygen species. Cell injury to myocytes in Krebs–Ringer bicarbonate buffer containing 5% fetal bovine serum was quantitated by measuring LDH release and calculating a cell injury index, as described in the text. (A) Concentrations of desferri-exochelin 784SM ranging from 6 to 20 μM reduced cell injury in a concentration-dependent manner when given simultaneously with 100 μM H2O2 for 4 hr (P < 0.001 by repeated measures ANOVA) (n = 9 in each group). Deferoxamine (200 μM) reduced injury if added 2 hr before exposure to H2O2 (P < 0.001), but was ineffective if added simultaneously with H2O2 (P = not significant). (B) Myocytes exposed to a xanthine/xanthine oxidase system were treated for 6 hr with simultaneous administration of 50 μM deferoxamine, 20 μM iron-loaded exochelin 784SM (Fe-EXO), 20 μM desferri-exochelin 784SM (D-EXO), or control medium. The desferri-exochelin markedly reduced cell injury (P < 0.001), but deferoxamine and the iron-loaded exochelin had no effect (P = not significant) (n = 3).
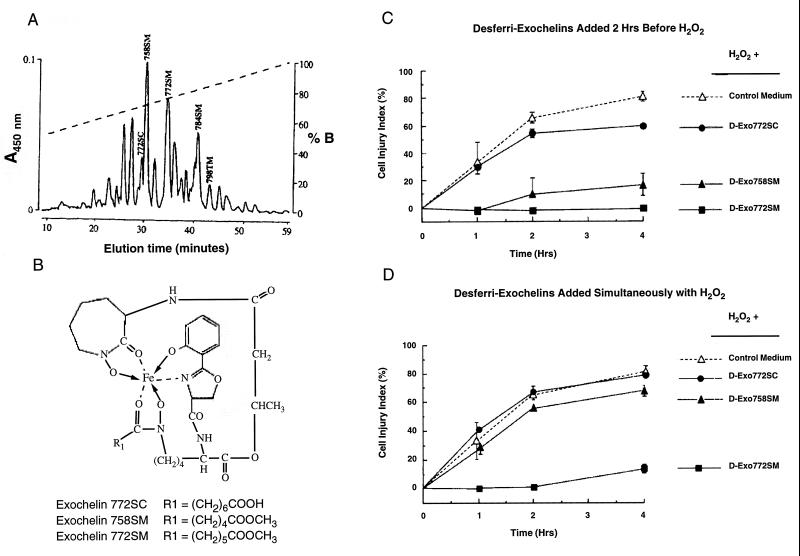
Lipid solubility would be expected to enhance diffusion of desferri-exochelins into cells and to enable them to chelate iron at key intracellular sites, including the lipid portions of the cell membrane. To test this hypothesis, we compared the protective efficacy of relatively polar (less lipid soluble) and nonpolar (more lipid soluble) desferri-exochelins in cardiac myocytes exposed to H2O2. When cardiac myocytes were treated with three different desferri-exochelins beginning 2 hr before exposure to H2O2, all reduced injury, but the magnitude of the protection depended on their lipid solubility (Fig. 3). The least lipid soluble desferri-exochelin (772SC) was least effective, the most lipid soluble (772SM) was most effective, and the desferri-exochelin of intermediate lipid solubility (758SM) exhibited a protective effect that was intermediate between the other two. When we added the same desferri-exochelins to cardiac myocytes simultaneously with exposure to H2O2, only the most lipid-soluble desferri-exochelin (772SM) exhibited a strong protective effect. The desferri-exochelin of intermediate solubility was minimally protective, and the least soluble exochelin was ineffective. Thus, the more lipid soluble the desferri-exochelin, the more it protected against H2O2-induced cell injury.
Figure 3.
Lipid solubility determines the capacity of desferri-exochelins to enter cells and protect against oxidant injury. (A) The elution profile of the exochelins present in the filtrate of a 6-week culture of the virulent Erdman strain of M. tuberculosis, using a reverse-phase HPLC C-18 column and an acetonitrile gradient. Each 450-nm absorbance peak contains an exochelin species whose mass and structure have been determined by mass spectrometry and tandem mass spectrometry (18). The mass in daltons of the iron-loaded form of each exochelin used in this study is labeled above the peak, followed by the letter S or T to indicate whether the exochelin is a member of the serine or threonine series and C or M to indicate whether its R1 side chain terminates with a carboxylic acid or methyl ester moiety. The dashed line represents the concentration of buffer B (50% acetonitrile/0.1% trifluoroacetic acid). The most polar molecules elute early (to the left) and the least polar (most soluble in lipid) elute late. (B ) The structures of three exochelins of different lipid solubility in the iron-loaded state. These three desferri-exochelins were added 2 hr before exposure to 100 μM H2O2 (C) and simultaneously with H2O2 exposure (D) (n = 3 for each group). The cultured cardiac myocytes were studied under the same conditions as in Fig. 2. The most polar desferri-exochelin, 772SC, slightly attenuated injury when added before H2O2 exposure (P < 0.01 by ANOVA with group as a nominal variable) but had no effect (P = not significant) when added simultaneously with H2O2. The most nonpolar (most lipophilic) desferri-exochelin, 772SM, was highly effective whether added 2 hr before or simultaneously with H2O2 exposure (P < 0.001 for each). The desferri-exochelin of intermediate polarity, 758SM, was moderately effective when added 2 hr before (P < 0.01) and had a very small, but significant, effect when added simultaneously with H2O2 exposure (P < 0.05).
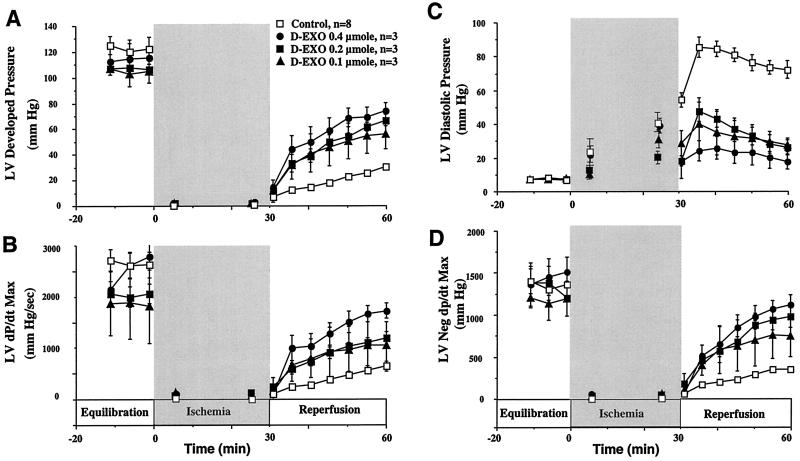
We then examined the protective effect of desferri-exochelins on isolated, perfused rabbit hearts subjected to ischemia and reperfusion (Fig. 4). Because relatively large quantities of exochelins were needed, we used mixtures of four nonpolar desferri-exochelins. Groups of rabbit hearts subjected to 30 min of global ischemia and 30 min of reperfusion were treated with a range of doses of desferri-exochelins administered into the root of the aorta during the first 2 min of reperfusion, whereas control hearts received only vehicle (saline). Treatment with desferri-exochelins markedly improved two indices of systolic function (developed pressure and dP/dt max) and two indices of diastolic function (ventricular end diastolic pressure and negative dP/dt max) in a dose-dependent manner (Fig. 4). In addition, treatment with desferri-exochelins reduced release of LDH into the coronary effluent, an index of myocardial necrosis, by approximately 20% (P < 0.02) (data not shown).
Figure 4.
Desferri-exochelins protect against cardiac reperfusion injury. Adult rabbit hearts were perfused by a nonrecirculating Langendorff technique with ventricular pacing. The hearts were subjected to 30 min of hypoxia followed by 30 min of reperfusion with oxygenated buffer. Mixtures of desferri-exochelins in saline (770SM, 772SM, 784SM, and 798TM) in doses of 0.1, 0.2, or 0.4 μmol, resulting in concentrations of approximately 1, 2, and 4 μM, (n = 3 for each dose) were injected into the root of the aorta during the first 2 min of reperfusion; the control group (n = 6) received saline. Values are mean ± standard error. In hearts treated with desferri-exochelins there was a dose-dependent improvement in two indices of left ventricular systolic function, developed pressure and dP/dt Max, during reperfusion (A and B) (P < 0.001 for each by repeated measures ANOVA). In addition, desferri-exochelins attenuated the deleterious elevation in left ventricular diastolic pressure during reperfusion (C) and improved (increased) another index of diastolic dysfunction, maximum negative dP/dt, in a dose-dependent manner (D) (P < 0.001 for each).
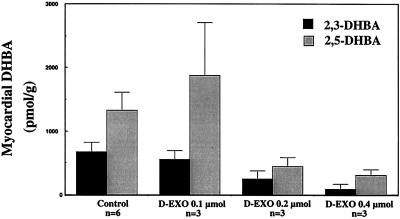
To confirm that the protective effect of desferri-exochelins was related to decreased ⋅OH formation in the rabbit hearts, we measured myocardial concentrations of hydroxylated salicylate isomers. In hearts treated with desferri-exochelins, there was a dose-dependent reduction in myocardial 2,3-DHBA and a strong trend toward reduction in 2,5-DHBA (Fig. 5). The 2,3-isomer is a more specific index of hydroxylation (19) and, because of slower tissue release, it is more reliable for measurement of myocardial concentrations.
Figure 5.
Desferri-exochelins (D-EXO) inhibit ⋅OH generation in hearts exposed to ischemia and reperfusion. Myocardial 2,3- and 2,5-DHBA were measured in the isolated hearts described in Fig. 4. Desferri-exochelins reduced 2,3-DHBA in a dose-dependent manner (P < 0.01 by linear regression). The dose-related change in 2,5-DHBA was at the margin of significance (P = 0.08). Because of its specificity for hydroxylation in tissue (21) and its greater retention in myocardium, the myocardial 2,3-DHBA is a more accurate index of ⋅OH production.
DISCUSSION
Although there are many iron chelators, most do not prevent iron-catalyzed conversion of H2O2 and ⋅O2− to more toxic ⋅OH (7). Desferri-exochelins prevent ⋅OH production and rapidly diffuse into cells where they prevent oxidant injury. Deferoxamine also prevents ⋅OH production but can enter cells only slowly by pinocytosis (15), and, therefore, is less attractive than desferri-exochelins for treatment of reperfusion injury. In addition, deferoxamine preincubated with cultured cardiac myocytes for sufficient time to eliminate delayed entry into the cells, had only one-tenth the potency of desferri-exochelins on a molar basis.
The superiority of desferri-exochelins appears to be because of their lipid solubility. Whereas deferoxamine is soluble only in water, desferri-exochelins are soluble in both water and lipid. A comparison of the protective effects against H2O2-induced injury of three desferri-exochelins with different lipid solubilities demonstrated that the most lipid soluble is the most potent and the most rapidly effective. Although exochelins share a common core structure about the iron binding site (18), each differs in polarity, depending primarily on variation in the length of the major alkyl side chain and whether the side chain terminates in a methyl ester or carboxylic acid moiety (18). Because polarity does not influence iron binding affinity (19), the differences among exochelins that we observed are almost certainly caused by differences in their lipid solubility.
Lipid solubility confers two important advantages on desferri-exochelins. First, it allows them to rapidly enter cells, probably because of the high phospholipid content of cell membranes. Second, it causes them to localize within the lipid compartments of the cell, which are major targets of oxidant reactions (10). If the iron molecules that are most critical to oxidant injury are localized in lipid compartments, this could explain the remarkable efficacy of extremely low concentrations of lipid-soluble desferri-exochelins. In addition to preventing iron-catalyzed conversion of H2O2 and ⋅O2− to ⋅OH, it is possible that desferri-exochelins suppress aerobic metabolism and the production of endogenous oxidants by removing iron from iron-centered enzymes.
We have demonstrated that desferri-exochelins, infused into the root of the aorta early in reperfusion, are capable of dramatically reducing cardiac injury in a dose-dependent manner. There was a strong association between suppression of ⋅OH accumulation in the myocardium by iron chelating desferri-exochelins and prevention of myocardial injury during reperfusion. In unpublished studies, we have found that exochelins bind trivalent cations (Fe3+, Ga3+, and Al3+) but not divalent cations (Ca2+, Mg2+, and Mn2+), or monovalent cations (Na+ and K+). These results support the hypothesis that iron-mediated formation of ⋅OH underlies reperfusion injury in the myocardium. Therefore, these lipid-soluble iron chelators potentially could be administered into the coronary circulation during reperfusion to reduce the injury that occurs after transient ischemia.
Acknowledgments
We thank Dr. Alastair D. Robertson for his assistance with statistical analysis. This work was supported by Grants HL55291, AI35725, and DK48520 from the National Institutes of Health.
ABBREVIATIONS
- LDH
lactic dehydrogenase
- FeNTA
ferric nitrilotriacetate
- DHBA
dihydroxybenzoic acid
References
- 1.Horwitz L D, Fennessey P V, Shikes R H, Kong Y. Circulation. 1994;89:1792–1801. doi: 10.1161/01.cir.89.4.1792. [DOI] [PubMed] [Google Scholar]
- 2.Connolly E S, Jr, Winfree C J, Springer T A, Naka Y, Liao H, Yan S D, Stern D M, Solomon R A, Gutierrez-Ramos J-C, Pinsky D J. J Clin Invest. 1996;97:209–216. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCord J M. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 4.Zweier J L, Flaherty J T, Weisfeldt M L. Proc Natl Acad Sci USA. 1984;184:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz L D, Leff J A. J Mol Cell Cardiol. 1995;27:909–915. doi: 10.1016/0022-2828(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 6.Floyd R A, Lewis C A. Biochemistry. 1983;22:2645–2649. doi: 10.1021/bi00280a008. [DOI] [PubMed] [Google Scholar]
- 7.Graf E, Mahoney J R, Bryant R G, Eaton J W. J Biol Chem. 1984;259:3620–3624. [PubMed] [Google Scholar]
- 8.Zweier J L. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 9.Lesnefsky E J, Ye J. Am J Physiol. 1994;266:H384–H392. doi: 10.1152/ajpheart.1994.266.2.H384. [DOI] [PubMed] [Google Scholar]
- 10.Kong Y, Lesnefsky E J, Ye J, Horwitz L D. Am J Physiol. 1994;267:H2371–H2377. doi: 10.1152/ajpheart.1994.267.6.H2371. [DOI] [PubMed] [Google Scholar]
- 11.Lesnefsky E J, Dauber I M, Horwitz L D. Circ Res. 1991;68:605–613. doi: 10.1161/01.res.68.2.605. [DOI] [PubMed] [Google Scholar]
- 12.Link G, Pinson A, Hershko C. J Lab Clin Med. 1993;121:127–134. [PubMed] [Google Scholar]
- 13.Ambrosio G, Zweier J L, Jacobus W E, Weisfeldt M L, Flaherty J T. Circulation. 1987;76:906–915. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- 14.Summers M R, Jacobs A, Tudway B, Perera P, Ricketts C. Br J Haematol. 1979;42:547–555. doi: 10.1111/j.1365-2141.1979.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd J B, Cable H, Rice-Evans C. Biochem Pharmacol. 1991;41:1361–1363. doi: 10.1016/0006-2952(91)90109-i. [DOI] [PubMed] [Google Scholar]
- 16.Reddy B R, Kloner R A, Przyklenk K. Free Radical Biol Med. 1989;7:45–52. doi: 10.1016/0891-5849(89)90099-3. [DOI] [PubMed] [Google Scholar]
- 17.Lesnefsky E J, Repine J E, Horwitz L D. J Pharm Exp Ther. 1990;253:1103–1109. [PubMed] [Google Scholar]
- 18.Gobin J, Moore C H, Reeve J R, Jr, Wong D K, Gibson B W, Horwitz M A. Proc Natl Acad Sci USA. 1995;92:5189–5193. doi: 10.1073/pnas.92.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gobin J, Horwitz M A. J Exp Med. 1996;183:1527–1532. doi: 10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byler R M, Sherman N A, Wallner J S, Horwitz L D. Am J Physiol. 1994;266:H121–H127. doi: 10.1152/ajpheart.1994.266.1.H121. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B, Kaur H, Ingelman-Sundberg M. Free Radical Biol Med. 1991;10:439–441. doi: 10.1016/0891-5849(91)90052-5. [DOI] [PubMed] [Google Scholar]