Abstract
Previous studies have shown chrysin, 7-hydroxyflavone and 7,4′-dihydroxyflavone to be the most potent flavonoid inhibitors of aromatase. However, very poor oral bioavailability is a major limitation for the successful use of dietary flavonoids as chemopreventive agents. We have recently shown that methylated flavones, including 5,7-dimethoxyflavone, 7-methoxyflavone and 7,4′-dimethoxyflavone, are much more resistant to metabolism than their unmethylated analogs and have much higher intestinal absorption. In this study, we examined these fully methylated flavones as potential aromatase inhibitors for the prevention and/or treatment of hormone-dependent cancers. Whereas 5,7-dimethoxyflavone had poor effect compared to its unmethylated analog chrysin, 7-methoxyflavone and 7,4′-dimethoxyflavone were almost equipotent to their unmethylated analogs with IC50 values of 2 to 9 μM. Thus, some fully methylated flavones appear to have great potential as cancer chemopreventive/chemotherapeutic agents.
Keywords: Aromatase inhibition, Flavonoids, Methylated flavones, Methoxyflavones
1. Introduction
The enzyme aromatase (cytochrome P450 (CYP)19), an important regulator of estrogen hormone availability, has become a target for new drug synthesis of inhibitors attempting to treat estrogen hormone-dependent cancers, which in addition to breast cancer [1] now also includes lung cancer [2].
Some naturally-occurring flavonoids, in particular chrysin (5,7-dihydroxyflavone), have also been shown in vitro to be aromatase inhibitors [3]. This gave rise to claims of chrysin as a booster of testosterone levels, leading to its marketing by health food stores and use by body builders. However, there is no support for its effectiveness in vivo. A clinical study demonstrated that the oral bioavailability of chrysin was much too low for any biological activity [4]. Another clinical study did not show any effect of chrysin on urinary testosterone levels [5]. Similar findings were made in a rat study [6].
In contrast, we have recently described high metabolic stability in the human liver as well as high intestinal transport of fully methylated flavones compared to the unmethylated analogs [7, 8] to predict high oral bioavailability. These methylated compounds, thus, have the potential to be effective aromatase inhibitors in humans in vivo.
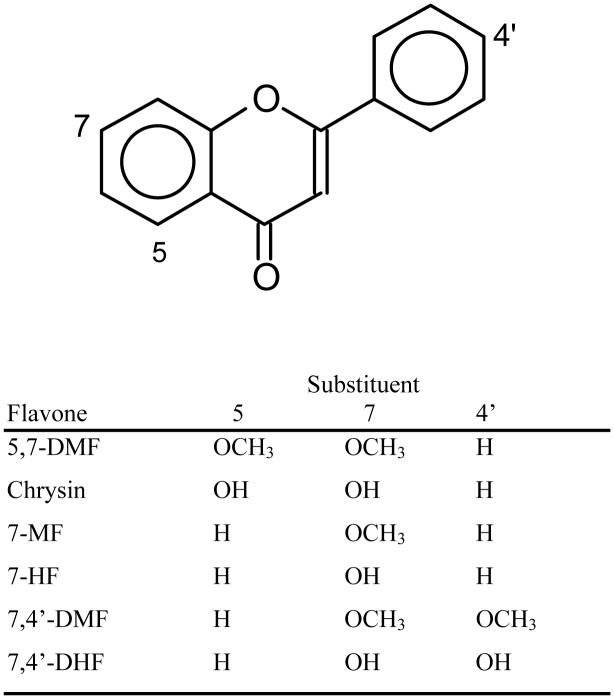
In the present study, we therefore determined the aromatase inhibitory activity of selected methylated flavones (see structures in Fig. 1). We compared the effects of the methylated versus the corresponding unmethylated analogs, the latter previously investigated by Ibrahim and Abul-Hajj [9]. The results suggest that some of these metabolically stable flavones may be effective aromatase inhibitors in humans in vivo.
Fig. 1.
Structures of flavones used in this study.
2. Materials and methods
Chrysin was obtained from Sigma Chemical Co. (St. Louis, MO). 5,7-Dimethoxyflavone (5,7-DMF), 7,4′-dimethoxyflavone (7,4′-DMF), 7,4′-dihydroxyflavone (7,4′-DHF), 7-methoxyflavone (7-MF) and 7-hydroxyflavone (7-HF) (structures, see Fig. 1) were obtained from Indofine Chemical Co., Inc. (Hillsborough, NJ).
The inhibition of aromatase (cytochrome P450 19) by the test flavones was investigated using a kit from Gentest (Woburn, MA) with recombinant CYP19 Supersomes as the enzyme source and dibenzylfluorescein as the substrate [10, 11] in a 96-well format. Serial dilutions of flavones (0.05-100 μM final concentrations) were preincubated at 37°C for 10 min with an NADPH generating system with control protein (0.1 mg/ml) in phosphate buffer. The enzymatic reaction was then carried out in the presence of 4 nM aromatase and 0.4 μM substrate for 30 min while shaking. The reaction was terminated with NaOH and the fluorescence was read 2 hr later in a plate reader with excitation at 485 nm and emission at 520 nm. Each flavone concentration was assayed in triplicates with appropriate background subtraction and controls.
Data were expressed as means ± SEM. Statistical significance of differences between samples were calculated by ANOVA with Dunnett multiple comparison post-test. P < 0.05 was considered significant. The IC50 values were calculated using Prism 4 (GraphPad Software, San Diego, CA).
3. Results and Discussion
The effect of the flavones in this study on aromatase activity used recombinant CYP19 as the enzyme source and a substrate that showed fluorescence upon metabolism.
Chrysin was a potent aromatase inhibitor with an IC50 of 4.2 μM (Fig. 2A), consistent with previous studies showing values of 0.5 to 2.6 μM [3]. The methylated analog, 5,7-DMF, showed very poor effect with an estimated IC50 of 123 μM (Fig. 2A). The flavone with the single hydroxyl group in the 7-position (7-HF) had previously been shown to be the most potent flavone inhibitor (IC50 0.50 μM) [9]. We found identical potency for 7-HF (IC50 0.51 μM) (Fig. 2B). In contrast to 5,7-DMF, 7-MF, i.e. the methylated analog of 7-HF, was only slightly less potent than 7-HF (Fig. 2B) with an IC50 value of 1.9 μM. 7,4′-DHF had an IC50 value of 3.2 μM, similar to the previously reported value of 2.0 μM [9], while its methylated analog 7,4′-DMF had an IC50 value of 9.0 μM.
Fig. 2.
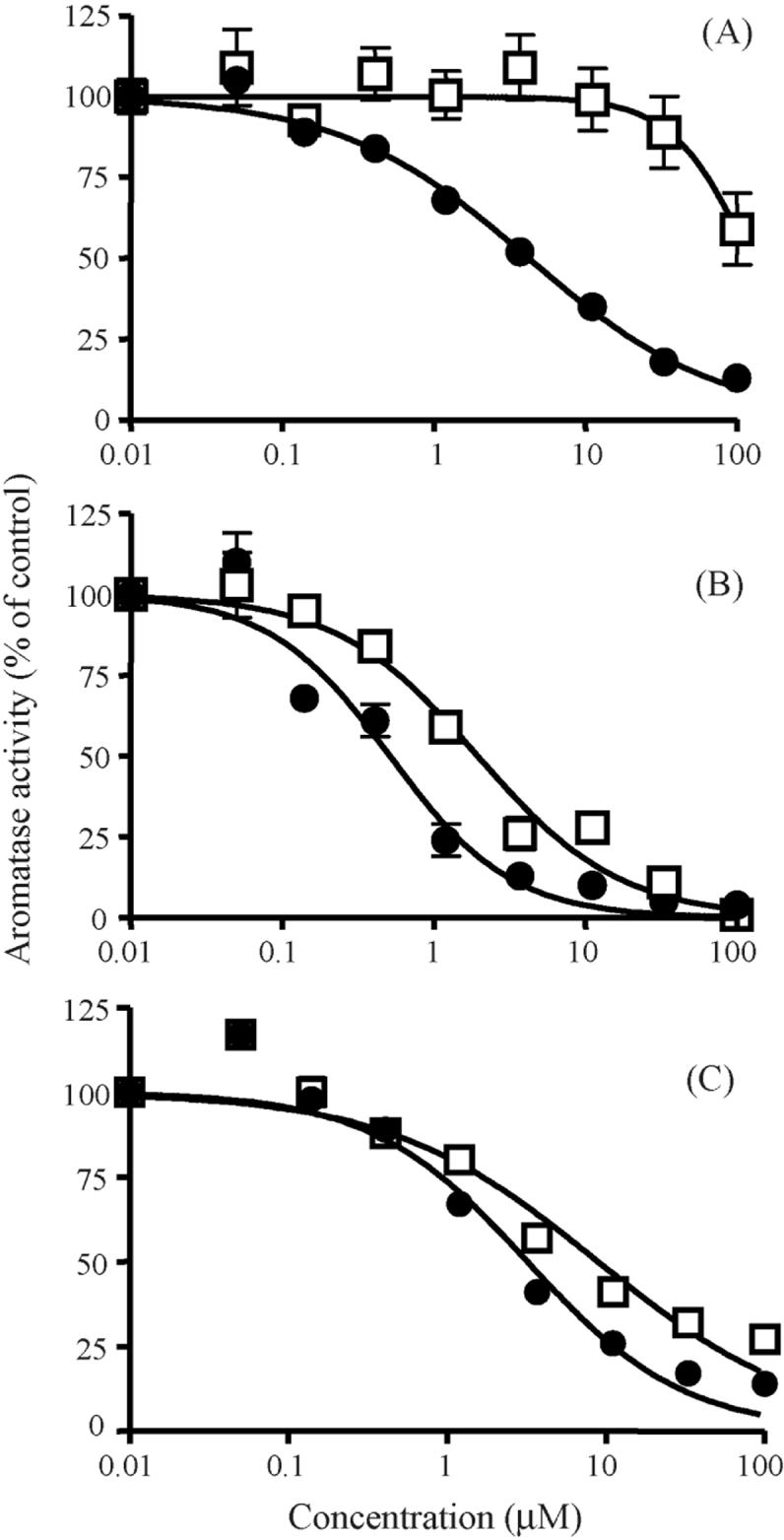
Effect of 5,7-DMF compared to chrysin (A), 7-MF compared to 7-HF (B), and 7,4′-DMF compared to 7,4′-DHF (C) on aromatase activity. □, methylated flavones; ●, unmethylated flavones. The data are expressed as percent of control. Mean values ± SEM (in many cases smaller than the symbols) are shown (n = 4 - 10).
The critical finding in this study is that two methylated flavones, 7,4′-DMF and especially 7-MF, were only slightly less potent than 7,4′-DHF and 7-HF, previously shown to be the two most potent flavone inhibitors of aromatase [9]. The importance of this finding lies in the fact that these methylated flavones are highly stable against human hepatic metabolism. In contrast, the unmethylated analogs, like chrysin, are very rapidly metabolized by sulfate and glucuronic acid conjugation [8]. In addition, in a human intestinal transport model, both 7-MF and 7,4′-DMF demonstrated high transport capacity compared to 7-HF and 7,4′-DHF [8]. The high metabolic resistance together with high rate of intestinal absorption would predict the two methylated flavones to be orally bioavailable in humans and thus capable of inhibiting aromatase in vivo. Further support for this contention is that 5,7-DMF but not chrysin has high oral bioavailability in rats [12].
Both 7-MF and 7,4′-DMF used in this study were synthetic compounds which could be used as food supplements or potentially as drugs. However, both are also found in plants. Thus, 7-MF has been found in extracts from Meliaceae and Rutaceae plants [13] and 7,4′-DMF has been identified in fruits and leaves from neotropical nutmeg species [14, 15] as well as from propolis [16].
Acknowledgements
This work was supported by the National Institutes of Health grant GM55561.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocr. Rev. 2005;26:331–45. doi: 10.1210/er.2004-0015. [DOI] [PubMed] [Google Scholar]
- [2].Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–91. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- [3].Kao Y-C, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ. Health Perspect. 1998;106:85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001;51:143–6. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gambelunghe C, Rossi R, Sommavilla M, Ferranti C, Rossi R, Ciculi C, Gizzi S, Micheletti A, Rufini S. Effects of chrysin on urinary testosterone levels in human males. J. Med. Food. 2003;6:387–90. doi: 10.1089/109662003772519967. [DOI] [PubMed] [Google Scholar]
- [6].Saarinen N, Joshi SC, Ahotupa M, Li X, Ammala J, Makela S, Santti R. No evidence for the in vivo activity of aromatase-inhibiting flavonoids. J. Steroid Biochem. Molec. Biol. 2001;78:231–9. doi: 10.1016/s0960-0760(01)00098-x. [DOI] [PubMed] [Google Scholar]
- [7].Wen X, Walle T. Methylation protects dietary flavonoids from rapid hepatic metabolism. Xenobiotica. 2006;36:387–97. doi: 10.1080/00498250600630636. [DOI] [PubMed] [Google Scholar]
- [8].Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006;34:1786–92. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- [9].Ibrahim A-R, Abul-Hajj YJ. Aromatase inhibition by flavonoids. J. Steroid Biochem. Molec. Biol. 1990;37:257–60. doi: 10.1016/0960-0760(90)90335-i. [DOI] [PubMed] [Google Scholar]
- [10].Stresser DM, Turner SD, McNamara J, Stocker P, Miller VP, Crespi CL, Patten CJ. A high-throughput screen to identify inhibitors of aromatase (CYP19) Anal. Biochem. 2000;284:427–30. doi: 10.1006/abio.2000.4729. [DOI] [PubMed] [Google Scholar]
- [11].Kragie L, Turner L, Patten C, Crespi C, Stresser D. Assessing pregnancy risks of azole antifungals using a high throughput aromatase inhibition assay. Endocr. Res. 2002;28:129–40. doi: 10.1081/erc-120015045. [DOI] [PubMed] [Google Scholar]
- [12].Walle T, Ta N, Kawamori T, Wen X, Tsuji PA, Walle UK.Cancer chemopreventive properties of orally bioavailable flavonoids - methylated versus unmethylated flavones Biochem. Pharmacol 2007. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ambrozin AR, Vieira PC, Fernades JB, da Silva MF, de Albuquerque S. Trypanocidal activity of Melicaceae and Rutaceae plant extracts. Mem. Inst. Oswaldo Cruz. 2004;99:227–31. doi: 10.1590/s0074-02762004000200020. [DOI] [PubMed] [Google Scholar]
- [14].Cavalcante SH, Fernandes D, Paulino Fo HF, Yoshida M, Gottlieb OR. Lignoids from the fruit of three Virola species. Phytochemistry. 1985;24:1865–6. [Google Scholar]
- [15].Santos LS, Corréa MJC, Campos LMO, Andrade MA. Constituents from the leaves of Virola michelli. Fitoterapia. 1996;67:555–6. [Google Scholar]
- [16].Popravko SA, Gurevich AI, Kolosov MN. Flavonoid components of propolis. Khimiya Prirodnykh Soedinenii. 1969;5:476–82. [Google Scholar]