Abstract
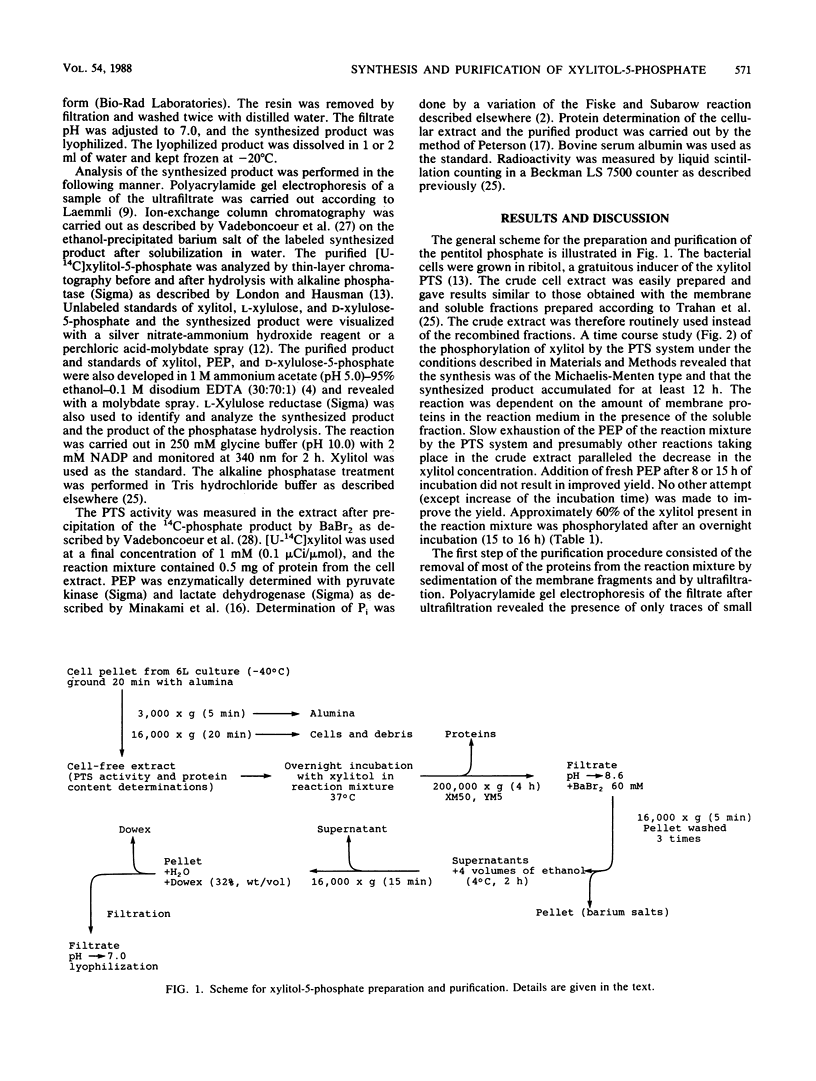
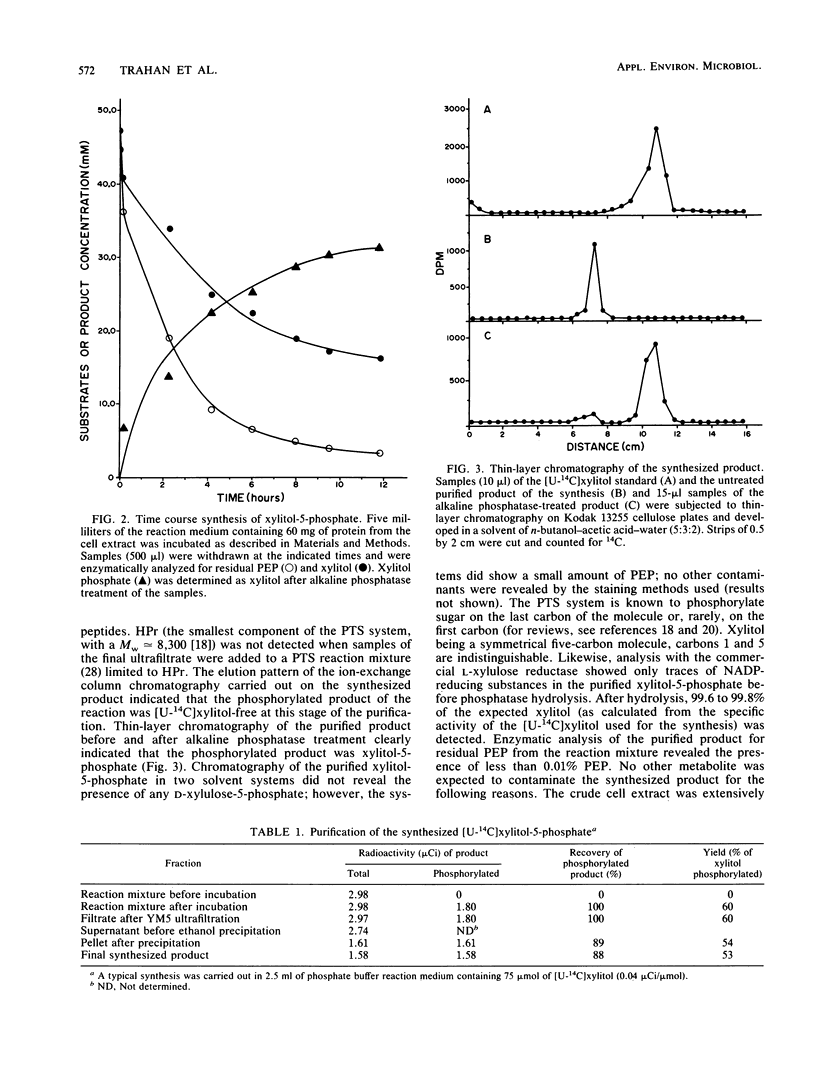
A simple procedure which yields pure xylitol-5-phosphate is described. A cell extract of Lactobacillus casei Cl-16 from a 6-liter culture was used to synthesize up to 70 mg of xylitol-5-phosphate overnight from xylitol and phosphoenolpyruvate via a xylitol phosphoenolpyruvate:phosphotransferase system with a 53% yield. Centrifugation, filtration, precipitation as a barium salt, and ion-exchange batch chromatography permitted recovery of nearly 90% of the phosphorylated product synthesized. Thin-layer chromatography and enzymatic analysis indicated a purity level of more than 99%. The method was used to synthesize [U-14C]xylitol-5-phosphate, and it is suitable for the synthesis of many other nonmetabolizable sugar phosphates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assev S., Vegarud G., Rölla G. Growth inhibition of Streptococcus mutans strain OMZ 176 by xylitol. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):61–63. doi: 10.1111/j.1699-0463.1980.tb02605.x. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. Properties of a Streptococcus lactis strain that ferments lactose slowly. J Bacteriol. 1984 Jan;157(1):28–34. doi: 10.1128/jb.157.1.28-34.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- Grahnén H., Myrberg N., Ollinen P. Fluoride and dental age. Acta Odontol Scand. 1975;33(1):1–4. doi: 10.3109/00016357509004620. [DOI] [PubMed] [Google Scholar]
- Hausman S. Z., London J. Purification and characterization of ribitol-5-phosphate and xylitol-5-phosphate dehydrogenases from strains of Lactobacillus casei. J Bacteriol. 1987 Apr;169(4):1651–1655. doi: 10.1128/jb.169.4.1651-1655.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman S. Z., Thompson J., London J. Futile xylitol cycle in Lactobacillus casei. J Bacteriol. 1984 Oct;160(1):211–215. doi: 10.1128/jb.160.1.211-215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenaar R., Huis in 't Veld J. H., de Stoppelaar J. D., Dirks O. B. Anti-cariogenic and remineralizing properties of xylitol in combination with sucrose in rats inoculated with Streptococcus mutans. Caries Res. 1984;18(3):269–277. doi: 10.1159/000260776. [DOI] [PubMed] [Google Scholar]
- Knuuttila M. L., Mäkinen K. Effect of xylitol on the growth and metabolism of Streptococcus mutans. Caries Res. 1975;9(3):177–189. doi: 10.1159/000260156. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach S. A., Green R. M. Effect of xylitol-supplemented diets on the progression and regression of fissure caries in the albino rat. Caries Res. 1980;14(1):16–23. doi: 10.1159/000260429. [DOI] [PubMed] [Google Scholar]
- London J., Chace N. M. New pathway for the metabolism of pentitols. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4296–4300. doi: 10.1073/pnas.74.10.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K. Biochemical principles of the use of xylitol in medicine and nutrition with special consideration of dental aspects. Experientia Suppl. 1978;(30):1–160. doi: 10.1007/978-3-0348-5757-4. [DOI] [PubMed] [Google Scholar]
- Mäkinen K. K. New biochemical aspects of sweeteners. Int Dent J. 1985 Mar;35(1):23–35. [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol. 1977 Oct;132(1):166–173. doi: 10.1128/jb.132.1.166-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T. The enzymology of the bacterial phosphoenolpyruvate-dependent sugar transport systems. Mol Cell Biochem. 1982 Jul 7;46(1):3–24. doi: 10.1007/BF00215577. [DOI] [PubMed] [Google Scholar]
- Scheinin A., Bánóczy J. Xylitol and caries: the collaborative WHO oral disease preventive programme in Hungary. Int Dent J. 1985 Mar;35(1):50–57. [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Regulation and function of sucrose 6-phosphate hydrolase in Streptococcus mutans. Infect Immun. 1979 Nov;26(2):487–491. doi: 10.1128/iai.26.2.487-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahan L., Bareil M., Gauthier L., Vadeboncoeur C. Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans. Caries Res. 1985;19(1):53–63. doi: 10.1159/000260829. [DOI] [PubMed] [Google Scholar]
- Trahan L., Mouton C. Selection for Streptococcus mutans with an altered xylitol transport capacity in chronic xylitol consumers. J Dent Res. 1987 May;66(5):982–988. doi: 10.1177/00220345870660052301. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Mayrand D., Trahan L. A comparative study of enzymes involved in glucose phosphorylation in oral streptococci. J Dent Res. 1982 Jan;61(1):60–65. doi: 10.1177/00220345820610011401. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M., Trahan L. Purification of proteins similar to HPr and enzyme I from the oral bacterium Streptococcus salivarius. Biochemical and immunochemical properties. Can J Microbiol. 1983 Dec;29(12):1694–1705. doi: 10.1139/m83-260. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Trahan L., Mouton C., Mayrand D. Effect of xylitol on the growth and glycolysis of acidogenic oral bacteria. J Dent Res. 1983 Aug;62(8):882–884. doi: 10.1177/00220345830620080601. [DOI] [PubMed] [Google Scholar]


