Abstract
Peripheral nerve injury markedly regulates expression of neurotrophins and their receptors in the lesioned nerve. However, the role of endogenously produced neurotrophins in the process of nerve regeneration is unclear. Expression of a multifunctional neurotrophin, pan-neurotrophin-1 (PNT-1), was targeted to the peripheral nerves of transgenic mice by using a gene promoter that is specifically activated after nerve lesion but that is otherwise silent in all other tissues and during development. PNT-1 is a chimeric neurotrophin that combines the active sites of the neurotrophins nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 and binds and activates all known neurotrophin receptors. In adult transgenic mice, PNT-1 was highly expressed in transected but not in intact sciatic nerve. Morphometric analyses at the electron microscopy level showed increased and accelerated recovery of axon diameter of myelinated fibers in crushed peripheral nerves of transgenic mice compared with wild type. Examination of nerve bundles in target tissues indicated accelerated reinnervation of foot pad dermis and flexor plantaris muscle in transgenic mice. Moreover, transected sensory and motor axons of transgenic mice showed faster and increased return of neurophysiological responses, suggesting an accelerated rate of axonal elongation. Importantly, transgenic mice also showed a markedly ameliorated loss of skeletal muscle weight, indicating functional regeneration of motor axons. Together, these data provide evidence, at both the anatomical and functional levels, that neurotrophins endogenously produced by the lesioned nerve are capable of significantly accelerating the regeneration of both sensory and motor axons after peripheral nerve damage. In addition, our results indicate that exogenous PNT-1 administration may be an effective therapeutic treatment of peripheral nerve injuries.
Keywords: nerve growth factor, brain-derived neurotrophic factor, nerve regeneration, site-directed mutagenesis, transgenic mice
Lesion of peripheral nerves may cause permanent denervation with paralysis and disability in humans and represents a challenging problem in neurosurgery. A slow rate of nerve regeneration conspires together with atrophy and degeneration of denervated organs to increase the risk of permanent disability after peripheral nerve injury, emphasizing the importance of rapid and timely reinnervation to optimize organ viability. Axonal repair of mature neurons involves complex molecular and cellular interactions. Neurotrophic factors are secreted polypeptides that control the survival, differentiation, and maintenance of vertebrate neurons (1, 2). These activities of neurotrophic factors have elicited great interest in these molecules as possible therapeutic agents in neurodegenerative diseases and nerve damage. Despite intense research in the neurotrophic factor field, relatively little is known about their physiological roles during peripheral nerve regeneration, although the possible utility of exogenously applied neurotrophic factors to promote and enhance axonal regrowth has been documented. Part of the difficulty in assessing the role of individual neurotrophic factors in nerve regeneration is because of the complex trophic requirements of sensory and motor neurons, which limit the efficacy achieved by any single factor.
The neurotrophins are a family of structurally and functionally related neurotrophic factors that play important roles in the development, maintenance, and functional plasticity of many neuronal populations in the central and peripheral nervous systems (3, 4). In vitro and in vivo studies, including gene knock-out experiments, have shown that different subpopulations of peripheral sensory neurons require different neurotrophins for survival and differentiation. Thus, nociceptive and thermoceptive sensory neurons in dorsal root ganglia (DRG) require nerve growth factor (NGF) (5), whereas muscle sensory neurons require neurotrophin-3 (NT-3) (6, 7). Although mice lacking any of the neurotrophins do not show deficits in motor neuron number during development, brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4) are potent survival and trophic factors for intact as well as injured motor neurons in vivo (8–12). Thus, sensory and motor neurons have heterogeneous neurotrophin requirements, and because their axons extend through the same peripheral nerves, functional regeneration after nerve injury is likely to require the concomitant action of different neurotrophins.
Expression of both neurotrophins and their receptors is regulated in response to peripheral nerve transection (13), suggesting that they form part of an endogenous mechanism of nerve repair. The functional importance of neurotrophins endogenously produced by the injured nerve in the process of nerve regeneration is, however, unclear. Much effort, on the other hand, has been devoted to study the effects of exogenously applied neurotrophins in lesioned peripheral nerves. NGF was shown to accelerate myelination and recovery of responsiveness to a noxious temperature stimulus after sciatic nerve transection (14). However, return of normal toe spread, a test of motor function, was not achieved by the NGF treatment (14). In another study, NGF was reported to have no effect on regeneration of axons from nociceptive neurons in injured thoracic cutaneous nerves (15). Similarly, NGF administration has been shown to prevent the behavioral and biochemical manifestations of experimental diabetic sensory neuropathy (16). This treatment, however, failed to prevent the reduction of conduction velocity predominantly associated with degeneration of larger, faster-conducting axons from motor neurons and large-fiber sensory neurons, which normally respond to NT-3 but not to NGF. Accordingly, a recent study demonstrated rescue effects of NT-3 on axotomy-induced changes in sensory conduction velocity (17). Finally, a moderate improvement in the rate of regeneration of larger-diameter axons and functional recovery in a walking track test recently has been observed after continuous administration of BDNF to the transected sciatic nerve bridged by collagen tubulization (18). In another study, a single dose of BDNF had no effect (19), and muscle weight was not restored by either treatment (18, 19).
Based on the evidence accumulated on the distinct specificities of the different neurotrophins on sensory and motor neurons, we hypothesized that genetically engineered neurotrophins combining the specific activities of multiple family members could constitute potent and efficacious therapeutic agents for peripheral nerve regeneration (20). To test this idea, and to evaluate the role of endogenously produced neurotrophins in nerve regeneration, we have designed a strategy to assay the ability of a multifunctional neurotrophin, pan-neurotrophin-1 (PNT-1), to promote functional nerve regeneration in the lesioned sciatic nerve. PNT-1 is a chimeric neurotrophin engineered by combining active domains of NGF, BDNF, and NT-3 into an NT-3 backbone. PNT-1 binds to and activates all neurotrophin receptors (21), is able to rescue the survival of multiple subpopulations of embryonic peripheral neurons with different neurotrophic requirements (22), and shows robust retrograde transport to different subpopulations of DRG neurons after injection into the rat sciatic nerve (22).
MATERIALS AND METHODS
Generation of Transgenic Mice.
A construct including a 5.5-kb fragment of the fourth exon and upstream flanking region of the rat BDNF gene, the PNT-1 gene (21), and the last small intron and polyadenylation signals of the rabbit β-globin gene was used to generate transgenic mice as previously described (23). The frequency of transgenic mice obtained was 26.5%. A line showing the desired expression pattern of the transgene subsequently was bred for homozygosity at the transgenic locus.
RNase Protection Assay.
Extraction of total RNA and RNase protection assay were performed as previously described (12) by using antisense cRNA probes specific for PNT-1.
Crush/Freeze Injury of the Sciatic Nerve.
The left sciatic nerve was exposed at its mid-thigh portion in anesthetized mice and crushed three times (20 s each) using Dumont microforceps, the tips of which previously had been cooled in liquid nitrogen. This resulted in a combined crush and freeze injury of the sciatic nerve just below the origin of the hamstring branch.
Morphometric Analysis.
Ultra-thin transverse sections were collected and processed for electron microscopy according to standard procedures. A minimum of three areas from electron micrographs comprising 2,200 μm2 were randomly sampled and photographed. The perimeter and the major and minor diameter axes of the myelinated fiber (D) and the axon (d) were recorded for each fiber. Samples from at least three transgenic and three wild-type mice were analyzed at 3 weeks, 5 weeks, and 9 weeks after lesion. Because axon diameters in sciatic nerve do not follow a normal distribution, an unpaired nonparametric test (Mann–Whitney) was used to compare the median value of the diameters in the different groups (representing about 300 fibers in each group) (graphpad-prism software). In a similar manner, about 200 unmyelinated axons from both transgenic and wild-type animals 5 weeks after lesion were analyzed. For controls, specimens from three adult unoperated wild-type or transgenic mice were analyzed by using the same protocol. Semi-thin sections and plastic embedding were done according to standard procedures. The density of nerve bundles in the dermis was examined within a depth of 100 μm from the epidermis.
Sciatic Nerve Transection and Tubulization.
The left sciatic nerve was exposed and transected at the mid-thigh level in mice anesthetized with sodium pentobarbital. A 3-mm-long (for 5-mm bypass) or 7-mm-long (for 10-mm bypass) nerve segment was removed, and the nerve endings were sutured with two stitches by using 10–0 Nylon inside a 7-mm-long (for 5-mm bypass) or 12-mm-long (for 10-mm bypass) silicon tube of 0.5-mm internal diameter (Kunii, Japan).
Electrophysiology.
A stimulation bipolar silver electrode was placed under the operated nerve 3–5 mm from the proximal stitch of the bypass (control stimulation, set to 100%) or 3–5 mm from the distal stitch of the bypass (for measurement of regeneration). Both ventral and dorsal roots were transected close to the spinal cord. The negative side of a recording electrode was placed under the distal L5 or L6 ventral [for motor compound action potential (CAP)] or dorsal (for sensory CAP) root; the positive side of the recording electrode was placed in the proximal root. Compound action potential was obtained by averaging 20 supramaximal stimulations of 1 Hz frequency, 5–100 mA amplitude, and 0.01–0.5 ms duration.
RESULTS
Targeted Expression of PNT-1 in the Lesioned Sciatic Nerve of Transgenic Mice.
Expression of PNT-1 was targeted to the peripheral nerves of transgenic mice by coupling the PNT-1 gene to the fourth promoter of the rat BDNF gene (Fig. 1A). This promoter normally shows low or negligible levels of activity during development (24, 25), but it is dramatically activated in adult peripheral nerves after nerve transection (26). This strategy allowed us to achieve sustained local levels of PNT-1 in the lesioned nerve without the need of exogenous delivery systems and permitted the assessment of the direct effects of PNT-1 on the process of nerve regeneration without introducing differences in the developmental histories of transgenic and wild-type animals. A line of transgenic mice homozygous for the PNT-1 transgene was generated. As expected, expression of PNT-1 mRNA, assessed by RNase protection assay (RPA), could not be detected in the intact adult sciatic nerve (Fig. 1B). However, PNT-1 mRNA expression was strongly induced in the distal segment of the transected transgenic sciatic nerve 7 days after the lesion (Fig. 1B). No increase was seen in the contralateral side to the lesion at any time point (data not shown). PNT-1 mRNA could not be detected by RPA in any other tissue examined at any developmental stage (data not shown). These results were in agreement with previous observations made in transgenic mice overexpressing the chloramphenicol acetyltransferase (CAT) reporter gene under the control of the same promoter (26), which demonstrated restricted activity to lesioned peripheral nerves, presumably in Schwann cells. Using the CAT transgene, expression from this BDNF gene promoter has been detected 7 days after transection, with a peak between the second and 3rd week after lesion (26). In the regenerating tubulized nerve, transgene expression is back down to control levels between 8 and 10 weeks after transection (T.T., unpublished data).
Figure 1.
Delivery of PNT-1 to the lesioned sciatic nerve by overexpression in transgenic mice. (A) Schematic representation of the PNT-1 expression construct. Hatched bar, upstream sequences of the fourth promoter of the BDNF gene; solid bar, coding region of the PNT-1 gene; open bar, rabbit β-globin gene intron and polyadenylation sequences. (B) Expression of PNT-1 mRNA in liver, intact sciatic nerve, and 1-week-lesioned sciatic nerve of transgenic mice analyzed by RPA.
Accelerated Maturation of Injured Axons in PNT-1 Transgenic Mice.
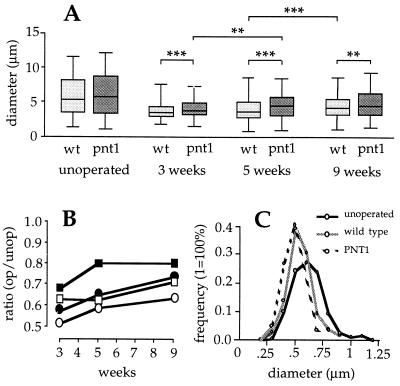
In the first set of experiments, sciatic nerves of transgenic and wild-type mice were lesioned by locally freezing a short nerve segment (about 3 mm) at the mid-thigh level by using a pair of forceps cooled by immersion in liquid N2. This procedure gave a very reproducible combined crush/freeze lesion that allowed the regeneration of axons across the lesioned segment. Portions of sciatic nerves distal to the site of injury were examined by electron microscopy 3, 5, and 9 weeks after the lesion. Five weeks after the lesion, operated nerves from wild-type and transgenic mice showed many of the morphological hallmarks of nerve degeneration, including infiltrating macrophages, signs of proliferation of Schwann cells and fibroblasts, reduced number and diameter of myelinated axons, and increased amounts of thin, unmyelinated fibers and cell debris, including myelin deposits devoid of axons (data not shown). A morphometric analysis was performed to compare the external diameters of myelinated axons of lesioned and intact nerves from wild-type and transgenic animals. Axon diameter correlates well with maturation in myelinated nerve fibers and constitutes a good morphological measure of the extent of axonal regeneration. Importantly, no significant differences were observed between nerves from unoperated wild-type and transgenic mice (Fig. 2A), indicating that the transgene did not introduce developmental differences between the two groups. In lesioned nerves, however, the external diameters of myelinated axons were significantly larger in transgenic than in wild-type mice at the three postoperation time points tested (P < 0.0001, P = 0.0003, and P = 0.002 at 3, 5, and 9 weeks, respectively, Mann–Whitney test) (Fig. 2A). Intriguingly, significant differences were also observed between transgenic and wild-type myelinated axons in the contralateral nerves of operated mice (data not shown). Although axon diameters in transgenic mice increased during the entire postoperation period investigated, regeneration was significantly more pronounced between the 3rd and 5th week (P = 0.0078, Mann–Whitney test) (Fig. 2A). In contrast, no significant differences could be seen during the same period in wild-type mice, in which the greatest increase in axon diameter occurred instead between the 5th and 9th week after the lesion (P < 0.0001, Mann–Whitney test) (Fig. 2A). Comparison of the ratios of operated to intact axon diameters in transgenic versus wild-type mice showed increased recovery of axon diameter in the transgenic group for both the median and upper 75 percentile values (Fig. 2B). Together, the results of these analyses indicated accelerated maturation of myelinated axons in nerves of transgenic mice overexpressing PNT-1.
Figure 2.
Morphometric analyses of myelinated and unmyelinated fibers in regenerating sciatic nerves of PNT-1 transgenic and wild-type mice. (A) Diameters of myelinated axons in unoperated and 3-, 5-, and 9-week postlesion sciatic nerves of wild-type (light gray) and transgenic (dark gray) mice. Boxes comprise the 25 and 75 percentiles, horizontal lines within boxes indicate the median, and smaller horizontal lines denote the highest and lowest values. Statistical comparisons between medians were made with an unpaired, nonparametric test (Mann–Whitney). ∗∗∗, P < 0.001; ∗∗, P < 0.01 (22). (B) Ratio of operated to intact axon diameters of the median (squares) and 75 percentile (circles) values in transgenic (solid) and wild-type (open) sciatic nerves at different times after lesion. (C) Size–frequency histogram of unmyelinated fibers from unoperated sciatic nerves (stippled line), and from lesioned wild-type (hatched line) and lesioned transgenic (solid line) sciatic nerves 5 weeks after lesion.
Morphological changes in unmyelinated fibers of regenerating nerves, comprising small C-fibers and sprouts, were also investigated. As expected, unmyelinated fibers from operated nerves of transgenic and wild-type mice were significantly smaller in diameter 5 weeks after the lesion than fibers from unoperated nerves (Fig. 2C). Although the peak of the size-distribution was identical in the wild-type and transgenic groups, a greater proportion of small unmyelinated fibers was observed in lesioned nerves of transgenic animals (P = 0.02, Mann–Whitney test) (Fig. 2C).
The total number of axons below the crush was estimated in sciatic nerves of wild-type and transgenic mice 5 and 9 weeks after the lesion. As expected (27, 28), the lesion had produced considerable sprouting in the nerves of both wild-type and transgenic animals. Five weeks after injury, the mean axon number in operated nerves of transgenic mice was 72% higher than in control unoperated nerves, whereas the corresponding figure in wild-type animals was 35% above the control value (n = 3). Nine weeks after the lesion, the mean axonal number in transgenic mice had decreased to 33% above control, whereas in wild-type animals it was still 50% higher than in unoperated nerves. These data indicated increased sprouting followed by accelerated elimination of sprouts in injured nerves expressing PNT-1.
Increased Reinnervation of Skin and Muscle After Nerve Injury in PNT-1 Transgenic Mice.
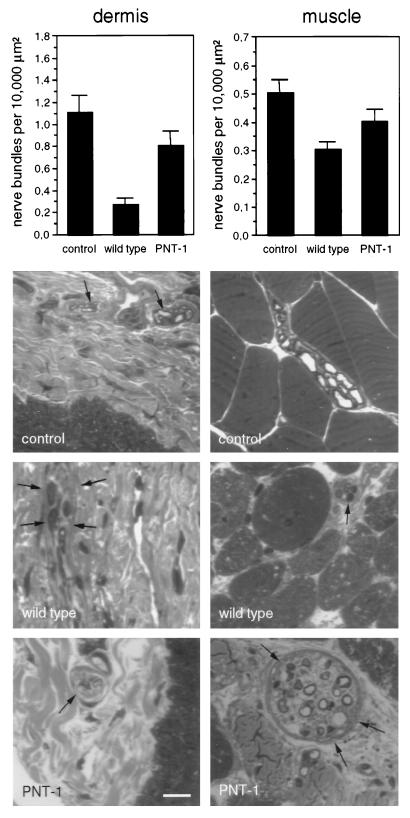
Skin and muscle reinnervation was examined in the freeze–crush paradigm. The density of axonal bundles was estimated in semi-thin sections of foot pad dermis and flexor plantaris muscle. Five weeks after lesion, axonal bundle density was increased in foot pad dermis of transgenic mice compared with wild type (Fig. 3 Left). A less pronounced increase was also seen in the number of axonal bundles in muscles of PNT-1 transgenic mice (Fig. 3 Right). In the latter case, regenerating muscular nerve bundles were considerably smaller in wild-type compared with transgenic animals (Fig. 3).
Figure 3.
Analyses of target reinnervation. The density of axonal bundles was estimated in semi-thin sections of foot pad dermis (Left) and flexor plantaris muscle (Right). In the upper graphs, the density of axonal bundles per 10,000 μm2 is indicated for unoperated nerves (control), and operated transgenic (PNT-1) and wild-type mice 5 weeks after nerve injury (n = 4). The lower micrographs show examples of semi-thin sections of dermis and muscle. Axonal bundles are indicated by arrows. (Bar = 10 μm.)
Accelerated Return of Neurophysiological Responses in Transected Sensory and Motor Nerves of PNT-1 Transgenic Mice.
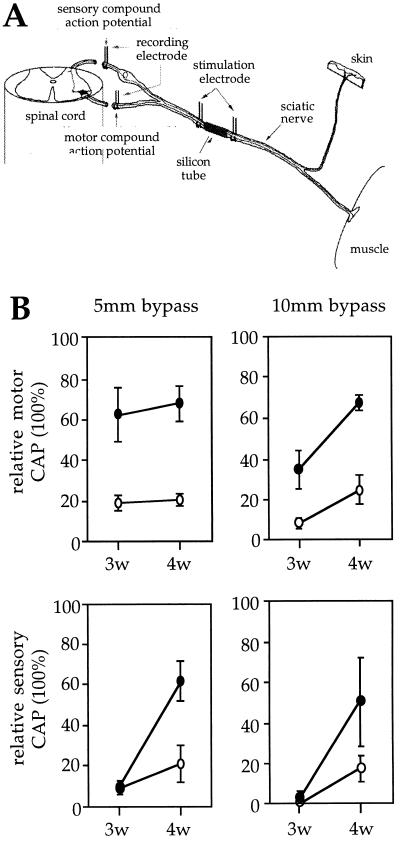
A truly multifunctional neurotrophin should be able to influence the rate of functional regeneration of both sensory and motor axons. Regeneration of PNT-1 transgenic and wild-type axons was assessed by electrophysiological recordings of CAPs in the L5-L6 dorsal (sensory regeneration) and ventral (motor regeneration) roots of transected sciatic nerves in which nerve gaps of either 5 or 10 mm had been bridged with a silicon tube. A quantitative measure was obtained as the percentage of the total area of CAPs assessed after electrical stimulation distally to the nerve lesion, relative to that obtained after proximal stimulation, which was arbitrarily set to 100% (Fig. 4A). Because the integration of CAPs is directly proportional to the number of axonal fibers traversing a nerve segment, the relative CAP is a measure of the extent of axonal elongation and/or branching across the gap. This analysis was performed in both the 5- and 10-mm bypass lesions, 3 and 4 weeks after nerve transection, which correspond to time points of maximal gene expression from BDNF promoter IV in transected sciatic nerves of transgenic mice (26). Three weeks after the lesion, motor axons in PNT-1 transgenic mice showed significantly higher levels of relative CAPs compared with wild type in both the 5- and 10-mm lesions (Fig. 4B). The differences between transgenic and wild-type animals were even greater 4 weeks after the lesion, when the relative CAPs in transgenic motor axons reached 68 and 70% recovery in the 5- and 10-mm lesions, respectively (Fig. 4B). Importantly, the slope of increase from the 3- to 4-week time points was higher in transgenic animals than in wild-type controls, indicating an acceleration of the rate of functional regeneration of motor axons in transgenic mice. A similar acceleration was seen in the rate of regeneration of sensory axons of transgenic mice. Although not significantly different 3 weeks after the lesion, the relative CAPs of sensory axons in both the 5- and 10-mm lesions were higher in PNT-1 transgenic than in wild-type mice 4 weeks after nerve transection (Fig. 4B). At this time point, the relative CAPs in transgenic sensory axons in the 5- and 10-mm lesions reached 61 and 52%, respectively (Fig. 4B). This level of recovery represented a 3-fold increase compared with that seen in wild-type animals at the same time point.
Figure 4.
Functional regeneration of motor and sensory axons in sciatic nerves of PNT-1 transgenic and wild-type mice. (A) Schematic representation of the strategy used for electrophysiological recordings of CAPs in dorsal and ventral roots. The total area of CAPs obtained after proximal stimulation was set to 100%. (B) Relative CAPs in lesioned motor (Upper) and sensory (Lower) axons of PNT-1 transgenic (solid circles) and wild-type (open circles) mice with a 5-mm (Left) or a 10-mm (Right) nerve bypass (n = 3; mean ± SEM).
Ameliorated Loss of Muscle Weight in PNT-1 Transgenic Mice.
A direct measure of functional regeneration of motor axons was obtained by assessing gastrocnemius muscle weight at different times after sciatic nerve transection and tubulization in the 10-mm bypass lesion. No difference in muscle weight was seen between unoperated transgenic and wild-type mice (data not shown). As expected, muscle mass in wild-type mice dropped to 35% of the unlesioned, contralateral side 2 weeks after nerve transection (Fig. 5). A similar decline was seen in gastrocnemius muscles of transgenic mice (Fig. 5). However, although muscle weight continued declining 3, 4, and 10 weeks after the lesion in wild-type animals, transgenic mice maintained muscle weight levels at 35–40% of the contralateral side for up to 10 weeks after nerve transection (Fig. 5). At this later time point, muscle weight in transgenic animals was almost twice that of wild-type controls (Fig. 5). Interestingly, the halt in the decline of muscle weight observed in transgenic animals coincided with the onset of maximal activity of BDNF promoter IV in the transected sciatic nerve (26). Muscle weight in the contralateral side was unchanged during the period examined and was indistinguishable between transgenic and control mice (data not shown).
Figure 5.
Time course of skeletal muscle weight decay in PNT-1 transgenic and wild-type mice. Gastrocnemius muscle weights of transgenic (solid circles) and wild-type (open circles) mice were determined 2, 3, 4, and 10 weeks after sciatic nerve transection (10-mm nerve bypass). Values are expressed as percentage of the weight of the contralateral muscle (n = 3; mean ± SEM).
DISCUSSION
In this study, we have investigated whether neurotrophins endogenously produced in the lesioned sciatic nerve may play a role in the process of nerve regeneration. To this end, we have studied the ability of the multifunctional neurotrophin PNT-1 to promote functional regeneration in the lesioned sciatic nerve of transgenic mice. PNT-1 is a chimeric neurotrophin that combines the specificities of NGF, BDNF, and NT-3 and efficiently activates all neurotrophin receptors. Local and sustained delivery of PNT-1 to the lesioned sciatic nerve was achieved by specifically targeting expression in transgenic mice in an injury-inducible manner, taking advantage of the unique properties of the BDNF gene promoter IV. Importantly, expression in the intact nerve was below detection levels. Accordingly, no differences could be seen in any of the parameters examined between unoperated transgenic and control mice, indicating that the developmental histories of transgenic and control mice were comparable. Because the PNT-1 transgene recapitulated the behavior previously seen for the CAT reporter gene with the same promoter in several lines of transgenic mice, we believe that the responses observed in nerve regeneration in the transgenic line studied here are attributable to the promoter-transgene and are unlikely to be the result of transgene integration site. Previous studies had demonstrated the ability of this promoter to target gene expression to Schwann cells (26). Schwann cells are known sources of a multitude of neurotrophic factors (29). In this regard, our data support to the role of Schwann cell-derived trophic factors in the process of peripheral nerve regeneration.
Targeted expression of PNT-1 to the lesioned sciatic nerve accelerated the recovery of several regeneration parameters including axon diameter, target reinnervation, neurophysiological responses, and muscle weight. The effect of PNT-1 on axon diameter was most evident in myelinated axons. A higher proportion of nonmyelinated fibers of smaller diameter was seen in lesioned nerves of transgenic mice that could have been due to increased sprouting induced by PNT-1 overexpression, a notion supported by the effects of PNT-1 on axon number and neurophysiological responses of regenerating nerves. Interestingly, sprouting in nerves of transgenic mice was followed by an accelerated elimination of sprouts, a process that is known to correlate with nerve maturation and target reinnervation (27, 28). In fact, an increased density of axonal bundles was observed in foot pad dermis and flexor plantaris muscle of transgenic mice compared with wild-type animals. That PNT-1 promoted regeneration of both sensory and motor axons was also suggested by the results of electrophysiological recordings of CAPs in dorsal and ventral roots, respectively. Finally, PNT-1 overexpression ameliorated muscle weight loss after nerve transection, an indication of functional motor regeneration. In a recent study, improvement of gastrocnemius muscle weight was observed after exogenous administration of NT-3 (30). The importance of preventing muscle atrophy cannot be overstated, because this is a crucial determinant of the functional outcome of the regeneration process. In this respect, it should be noted that neurotrophins, PNT-1 included, are unlikely to have a direct myotrophic effect, because no functional neurotrophin receptors are known to be expressed in skeletal muscle (H.F. and C.F.I., unpublished data). Instead, the reduced loss of muscle weight in transgenic mice is more likely to be a consequence of motor axon regeneration, although an indirect effect mediated via induction of a second, neuron-derived factor cannot be ruled out.
Previous studies indicated a dynamic pattern of expression of neurotrophins and their receptors after peripheral nerve lesion. Expression of the p75 neurotrophin receptor, NGF, BDNF, and NT-4 is induced in the distal segment of the transected sciatic nerve (13, 31). TrkB and TrkC neurotrophin receptor expression is also regulated after lesion (13), and in denervated gastrocnemius muscle, BDNF expression increases while that of NT-4 decreases (12, 13). However, the importance of this complex response for the process of axonal regeneration is not understood. Although gene knock-outs are available for all the neurotrophins, these animals die too early to allow examination of nerve regeneration responses. We have taken an alternative approach and used a gene promoter that is specifically activated after nerve lesion to boost the endogenous levels of neurotrophin activity in the lesioned nerves of transgenic mice. Our data provide evidence at both the anatomical and functional levels that neurotrophins endogenously produced by the lesioned nerve are capable of significantly accelerating the regeneration of both sensory and motor axons after peripheral nerve damage. The fact that levels of PNT-1 sufficient to promote nerve regeneration could be achieved by endogenous production indicates the feasibility of using gene therapy approaches for the delivery of neurotrophic proteins to the injured peripheral nervous system. Finally, our results lend support to the notion that multifunctional neurotrophins are potent and efficacious agents for peripheral nerve regeneration, and raise the possibility of the therapeutic use of PNT-1 in peripheral neuropathies and nerve injuries. Future studies should address the efficacy of exogenous PNT-1 application, the time window of PNT-1 therapy after lesion, and possible differential effects of PNT-1 on complete nerve transections versus simple crushes.
Acknowledgments
We thank Mike Fainzilber, Ernest Arenas, and Miles Trupp for comments on the manuscript. We also thank Eric Nilsson, Pellina Janson, and Annika Ahlsén for technical assistance and Lotta Johansson for secretarial help. Financial support was obtained from the Swedish Medical Research Council and the Karolinska Institute. H.F. was supported by fellowships from the Human Frontier Science Program Organization and from the Swedish Medical Research Council.
ABBREVIATIONS
- PNT-1
pan-neurotrophin-1
- DRG
dorsal root ganglia
- NGF
nerve growth factor
- NT
neurotrophin
- BDNF
brain-derived neurotrophic factor
- CAP
compound action potential
- CAT
chloramphenicol acetyltransferase
References
- 1.Ibáñez C F, Hökfelt T, Olson L, Fuxe K, Jörnvall H, Ottoson D. Wenner-Gren Center International Series. Vol. 67. Oxford: Elsevier; 1995. , 472 pp. [Google Scholar]
- 2.Barde Y-A. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 4.Lewin G, Barde Y-A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 5.Crowley C, Spencer S D, Nishimura M C, Chen K S, Pittsmeek S, Armanini M P, Ling L H, Mcmahon S B, Shelton D L, Levinson A D, Phillips H S. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 6.Ernfors P, Lee K F, Jaenisch R. Nature (London) 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 7.Fariñas I, Jones K R, Backus C, Wang X Y, Reichardt L F. Nature (London) 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheim R W, Yin Q W, Prevette D, Yan Q. Nature (London) 1992;360:755–759. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- 9.Yan Q, Elliott J, Snider W D. Nature (London) 1992;360:753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 10.Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde Y A. Nature (London) 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 11.Koliatsos V E, Clatterbuck R E, Winslow J W, Cayouette M H, Price D L. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibáñez C F. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 13.Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge V M K, Persson H. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derby A, Engleman V W, Frierdich G E, Neises G, Rapp S R, Roufa D G. Exp Neurol. 1993;119:176–191. doi: 10.1006/exnr.1993.1019. [DOI] [PubMed] [Google Scholar]
- 15.Diamond J, Foerster A, Holmes M, Coughlin M. J Neurosci. 1992;12:1467–1476. doi: 10.1523/JNEUROSCI.12-04-01467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apfel S C, Arezzo J C, Brownlee M, Federoff H, Kessler J A. Brain Res. 1994;634:7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 17.Munson J B, Shelton D L, Mcmahon S B. J Neurosci. 1997;17:470–476. doi: 10.1523/JNEUROSCI.17-01-00470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utley D S, Lewin S L, Cheng E T, Verity A N, Sierra D, Terris D J. Arch Otolaryngol Head Neck Surg. 1996;122:407–413. doi: 10.1001/archotol.1996.01890160047009. [DOI] [PubMed] [Google Scholar]
- 19.Shirley D M, Williams S A, Santos P M. Laryngoscope. 1996;106:629–632. doi: 10.1097/00005537-199605000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Ibáñez C F. Trends Biotechnol. 1995;13:217–227. doi: 10.1016/S0167-7799(00)88949-0. [DOI] [PubMed] [Google Scholar]
- 21.Ibáñez C F, Ilag L L, Murray-Rust J, Persson H. EMBO J. 1993;12:2281–2293. doi: 10.1002/j.1460-2075.1993.tb05882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilag L L, Curtis R, Glass D, Funakoshi H, Tobkes N, Ryan T, Acheson A, Lindsay R, Persson H, Yancopoulos G D, et al. Proc Natl Acad Sci USA. 1995;92:607–611. doi: 10.1073/pnas.92.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson E, Lendahl U. Mol Reprod Dev. 1993;34:149–157. doi: 10.1002/mrd.1080340206. [DOI] [PubMed] [Google Scholar]
- 24.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 25.Timmusk T, Belluardo N, Persson H, Metsis M. Neuroscience. 1994;60:287–291. doi: 10.1016/0306-4522(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 26.Timmusk T, Lendahl U, Funakoshi H, Arenas E, Persson H, Metsis M. J Cell Biol. 1995;128:185–199. doi: 10.1083/jcb.128.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramón y Cajal S, editor. Degeneration and Regeneration of the Nervous System. Oxford and New York: Oxford Univ. Press; 1928. [Google Scholar]
- 28.Sunderland S, editor. Nerves and Nerve Injuries. Edinburgh and New York: Churchill Livingstone; 1978. [Google Scholar]
- 29.Davies A M. Curr Biol. 1998;8:R15–R18. doi: 10.1016/s0960-9822(98)70009-0. [DOI] [PubMed] [Google Scholar]
- 30.Sterne G D, Coulton G R, Brown R A, Green C J, Terenghi G. J Cell Biol. 1997;139:709–715. doi: 10.1083/jcb.139.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson E M, Taniuchi M, DiStefano P S. Trends Neurosci. 1988;11:299–304. doi: 10.1016/0166-2236(88)90090-2. [DOI] [PubMed] [Google Scholar]