Abstract
The activity of chemical carcinogens is a complex balance between metabolic activation by cytochrome P450 monooxygenases and detoxification by enzymes such as glutathione S-transferase (GST). Regulation of these proteins may have profound effects on carcinogenic activity, although it has proved impossible to ascribe the observed effects to the activity of a single protein. GstP appears to play a very important role in carcinogenesis, although the precise nature of its involvement is unclear. We have deleted the murine GstP gene cluster and established the effects on skin tumorigenesis induced by the polycyclic aromatic hydrocarbon 7,12-dimethylbenz anthracene and the tumor promoting agent 12-O-tetradecanoylphorbol-13-acetate. After 20 weeks, a highly significant increase in the number of papillomas was found in the GstP1/P2 null mice [GstP1/P2(−/−) mice, 179 papillomas, mean 9.94 per animal vs. GstP1/P2(+/+) mice, 55 papillomas, mean 2.89 per animal, (P < 0.001)]. This difference in tumor incidence provides direct evidence that a single gene involved in drug metabolism can have a profound effect on tumorigenicity, and demonstrates that GstP may be an important determinant in cancer susceptibility, particularly in diseases where exposure to polycyclic aromatic hydrocarbons is involved, for instance in cigarette smoke-induced lung cancer.
Glutathione S-transferases (GSTs) are a superfamily of enzymes, responsible for the detoxification of a wide range of xenobiotics. These enzymes catalyze the nucleophilic attack of reduced glutathione (GSH) on electrophilic compounds and have evolved as a cellular protection system against their toxic effects (1, 2). On the basis of primary sequence similarity, mammalian cytosolic GST can be divided into five subclasses, alpha, mu, pi, theta, sigma, and zeta. Microsomal forms also have been characterized (3).
There has been considerable interest in the properties of the pi-class GST, particularly in relationship to carcinogenesis and human cancer. A number of studies have reported that pi-class GST is substantially elevated in the early stages of rat liver carcinogenesis (4–6). Indeed, this protein is one of the most reliable markers for the identification of hepatic preneoplastic foci. GST pi is almost undetectable in normal rat hepatocytes and is markedly overexpressed in hepatic foci arising spontaneously or in animals treated with carcinogens. (7, 8).
In humans, overexpression of pi-class GST has been associated with carcinogenesis and tumor development, as well as drug resistance (9–15). Expression of GSTP has been reported to be elevated in many human tumors, including lung (16), colon (7), ovary (17), testis (18), bladder (19), oral (20), and kidney (21). In several cases this has been correlated with malignancy and drug resistance, and inversely with patient survival (16, 22). Interestingly, in contrast to these data, it has been reported that in prostate cancer, GSTP expression is completely abolished because of hypermethylation of the promotor (23), indicating that GSTP has an important function in tumor development. A subsequent study (24) confirmed these findings but sought to explain the results by pointing out that GSTP is specifically expressed in the basal epithelium, a cell layer that is absent in malignant prostate tissue.
Our laboratory has been involved in the study of GSTP for a number of years (12, 25, 26), and much progress has been made in understanding the mechanisms of transcriptional regulation (27–29). However, despite intensive study, little is known about the role of GstP in carcinogenesis and drug resistance. To resolve this problem, we have deleted the GstP genes from the mouse and investigated the changes in sensitivity to polycyclic aromatic hydrocarbon (PAH)-induced skin carcinogenesis.
To carry out this work, it was necessary to characterize the organization of the GstP genes in this species. Bammler et al. (30) first reported cloning of two murine genes (GstP1 and GstP2), which lie in tandem, separated by approximately 2.5 kb of DNA. Both genes are actively transcribed, and their coding regions differ by only six amino acid residues, three of which (residues 10, 11, and 104) have been identified as being responsible for the profound differences in catalytic activities between the two proteins (31). In the mouse, expression of pi-class GST is sexually dimorphic, males having levels approximately an order of magnitude greater than females, and in addition, GstP1 is transcribed at much higher levels in both sexes (30). The detailed characterization of the structure of the GstP gene cluster, and the fact that there are only two such genes in the mouse, close together in the genome, has allowed us to apply gene-targeting technology to delete or inactivate both GstP genes simultaneously.
MATERIALS AND METHODS
Reagents.
All chemicals used in this study were of analytical grade or better and were obtained from Sigma or Merck. Restriction enzymes and plasmids were obtained from GIBCO, Stratagene, and Promega. Plasmid preparation, plasmid purification, and DNA isolation from agarose gels were carried out using kits from Qiagen.
Cloning of Murine GstP Genes and Construction of Targeting Vector.
A genomic library from 129 mice was screened with the mouse GstP1 cDNA, and a number of overlapping clones were isolated and characterized. A targeting vector was constructed by using the en2A-IRESβgeoPA cassette (32) to replace exons 6 and 7 of GstP2, the intergenic region, and the entire GstP1 gene, including approximately 1.5 kb of 3′ untranslated region (Fig. 1 A and B). This powerful strategy for selecting ES cell lines where homologous recombination has occurred depends on the expression of GstP2 in ES cells, which was confirmed by reverse transcription–PCR (not shown). The targeting construct was linearized with NotI purified and transfected into E14Tg2a.IV ES cells by electroporation, and colonies were selected for G418 resistance. Southern blotting of EcoRI- or KpnI-digested genomic DNA using DNA probes flanking the 5′ and 3′ untranslated regions of the GstP gene cluster identified integrants where homologous recombination had occurred (Fig. 1 C and D). A correctly targeted clone was expanded, grown up and injected into blastocysts, before being implanted in surrogate mothers. Resulting chimeric mice, in which the targeted ES cells had contributed to the genome, were identified by coat color, and crossed with the albino MF1 mouse strain. Offspring in which germ-line transmission of the targeted locus had occurred were identified by coat color, and the disrupted GstP locus confirmed by Southern blotting of tail-tip genomic DNA. Mice heterozygous for the targeted locus were crossed, and GstP1/P2(−/−), GstP1/P2(+/−), and GstP1/P2(+/+) mice identified by Southern blotting of genomic DNA using GstP-specific probes. Lines of GstP1/P2(−/−) mice, as well as nontransgenic littermates, were established by random crossing. Mice were initially kept in an isolator, before transferral to a specific pathogen-free facility, where they were fed ad libitum. All animal work was performed in accordance with the United Kingdom Animal Scientific Procedures Act (1986).
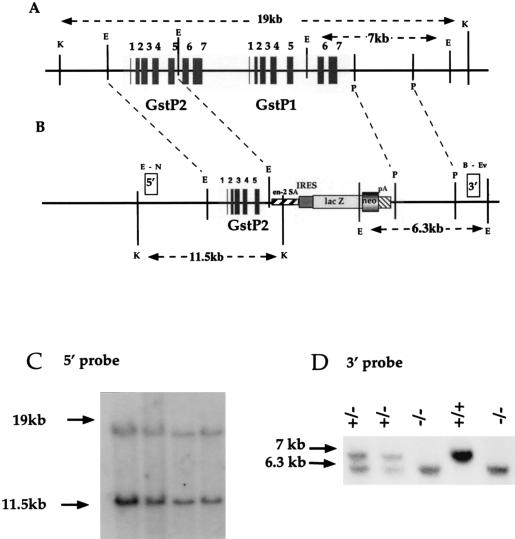
Figure 1.
Strategy for inactivation of the murine GstP gene cluster. (A) GstP1/P2 gene cluster and (B) targeted GstP1/P2 allele. Restriction endonucleases: B, BglII; E, EcoRI; Ev, EcoRV; K, KpnI; N, NheI; P, PstI. A cassette containing the en-2A splice acceptor site, internal ribosome binding site element, lacZ, neo (βGeo), and simian virus 40 polyadenylation site, replaces genomic DNA from the EcoRI site in GstP2, exon 5, to the first PstI site in the 3′ untranslated region of GstP1. Coding exons are represented by black boxes; white boxes represent the region of DNA used as 5′- and 3′-flanking probes for Southern screening. (C) Southern blot analysis of ES cell genomic DNA from targeted clones. The 19-kb KpnI fragment represents the wild-type allele, and the 11.5-kb band corresponds to the targeted allele, using the 5′-flanking probe. (D) Southern blot analysis of tail-tip genomic DNA from wild-type (+/+), heterozygous (+/−), and null (−/−) mice. The 7-kb EcoRI fragment represents the wild-type allele, whereas the 6.3-kb band corresponds to the targeted allele, using the 3′-flanking probe.
Mice were sacrificed by a rising concentration of CO2, before removal of organs. Tissues were rinsed in ice-cold sterile PBS and snap-frozen in liquid nitrogen and stored at −70°C until preparation of subcellular fractions.
DNA Sequencing and Southern blotting.
Sequencing of cloned genomic DNA or PCR-amplified sequences was carried by the dideoxy method of Sanger et al. (33).
Southern blots were carried out on genomic DNA, either from ES cells or from mouse tail-tips (approximately 10 μg) and were digested with 50–100 units of the appropriate restriction enzyme, overnight at 37°C. Southern blotting of these samples and labeling of DNA fragments by random priming was carried according to published procedures (34, 35). Briefly, the samples were run on a 0.4% agarose gel in 1× TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA), overnight at a low voltage to optimize separation of DNA fragments. The gel was denatured in 0.25 N HCl for 30 min, before being transferred, in a downward direction by capillary action, to Qiabrane Nylon+ (Qiagen) in 0.4 M NaOH for 2.5 h. The filter subsequently was rinsed in 2× SSC (20× SSC is 3 M NaCl, 0.3 M tri-sodium citrate), and prehybridized at 65°C in a solution containing 2× SSC, 1% SDS, 0.5% fat-free milk powder, and 0.75 mg/ml of denatured herring sperm DNA. After 2–4 h, this solution was replaced by a hybridization solution, containing essentially as for the prehybridization solution, except the herring sperm DNA was at 0.5 mg/ml, and the labeled, denatured DNA probe was added. Hybridization was continued for 16–24 h at 65°C, before the filter was washed with decreasing concentrations of SSC, containing 0.1% SDS, at 65°C. The filter was exposed to Kodak X-Omat XAR5 autoradiography film for varying lengths of time at −70°C with intensifying screens.
Preparation of Subcellular Fractions, Determination of Cytosolic GST Activity, and Immunoblotting.
Microsomal and cytosolic fractions were prepared from mouse tissues, and protein concentrations were determined, as described previously (34). Activity of the mouse cytosolic fractions toward 1-chloro-2,4-dinitrobenzene (CDNB) and ethacrynic acid were determined spectrophotometrically, using previously published protocols (36).
Western blots were carried out as described previously (37), using 9% (microsomal) or 12% (cytosolic) resolving gels on SDS/PAGE, and electro-transfer to nitrocellulose membranes. This study used polyclonal antisera raised to various rat or mouse GST (36, 38, 39) or rat P450 (40) and an anti-rabbit IgG-horseradish peroxidase second antibody obtained from the Scottish Antibody Production Unit, Carluke, United Kingdom. Immunoreactivity was determined by using a chemiluminescent method (ECL, Amersham) and Kodak XAR5 autoradiographic film. Loading equivalence was confirmed by staining SDS/PAGE gels with Coomassie blue-R.
Preparation of RNA and Northern Blotting.
RNA was prepared from mouse tissues, the concentration was estimated spectrophotometrically, and Northern blotting was performed as previously described (34), using Qiabrane Nylon+ membrane (Qiagen). Briefly, after RNA was separated on a denaturing agarose gel and transferred to the filter by capillary action, the filter was UV-crosslinked (Stratagene) and placed in prehybridization solution [2× Denhardt’s (1× Denhardt’s is 0.02% BSA, 0.02% polyvinylpyrrolidone, 0.02% Ficoll), 6× SSC, 0.1% (wt/vol) SDS, 0.1% (wt/vol) sodium pyrophosphate, 100 μg/ml of denatured herring sperm DNA] for 2–4 h at 65°C. Hybridization was carried out at 65°C for 16–24 h, in a solution essentially as for prehybridization, except omitting herring sperm DNA, and including dextran sulfate at a final concentration of 10% (wt/vol). when the filter was washed with decreasing concentrations of SSC, containing 0.1% SDS, at room temperature and 65°C. The filter was exposed to Kodak X-Omat XAR5 autoradiographic film for varying lengths of time at −70°C with intensifying screens.
Skin Carcinogenesis Study.
Female GstP1/P2(−/−) and GSTP1/P2(+/+) mice, aged 8–10 weeks old, were used in skin carcinogenesis experiments. The dorsal skin was shaved 24 h before exposure to the carcinogen 7,12-dimethylbenz anthracene (DMBA) (25 μg in 200 μl of acetone). The tumor promoting agent 12-O-tetradodecanoyl-13-acetate (TPA) then was applied twice a week (5 × 10−5M in 200 μl acetone; 10 nmol) beginning 1 week after initiation and continuing for an additional 20 weeks. The papilloma incidence was monitored weekly (41).
RESULTS
The targeting strategy used to delete the GstP genes leaves exons 1–5 of the GstP2 gene intact (Fig. 1A). To confirm that the remaining peptide could not constitute a functional protein, exons 1–5 of GstP2 were amplified by PCR, using Pfu polymerase (Stratagene), and cloned into a pet15b expression vector (Novagen). PCR errors were ruled out by dideoxy sequencing, and the expression plasmid was induced by exposure to isopropyl β-d-thiogalactoside for 24 h. Cells were harvested, and Western blotting of cell extracts was carried out, using an antibody raised to murine GstP. A single band representing the truncated GstP2 protein was clearly visible, however, no CDNB-conjugating activity was detectable in these cells (data not shown).
Electroporation of the GstP1/P2 deletion construct into ES cells and selection for neomycin resistance yielded 25 clones. Restriction enzyme digestion of DNA isolated from these clones was digested with EcoRI and Southern-blotted with the GstP-specific probes (Fig. 1 B-D), confirming that homologous recombination had occurred correctly in 16 of these clones. ES cells from one of the correctly targeted clones were injected into blastocysts, transferred into surrogate mothers, and allowed to develop to term. Chimeric mice were identified by coat color and crossed to MF1 mice, and offspring in which the targeted ES cells had contributed to the germ line were identified (also by coat color). Heterozygotes were crossed to generate GstP1/P2(−/−), GstP1/P2(+/−), and GstP1/P2(+/+) mice in mendelian proportions of 1:2:1, indicating that mice of any particular genotype were not being lost in utero. Colonies of GstP1/P2(−/−) and GstP1/P2(+/+) mice were maintained by random crossing to avoid problems with genetic background.
GstP null mice appeared healthy, with no obvious signs of distress or illness. Gross examination of major organs revealed no abnormalities, which was confirmed by histopathological examination of sections from a variety of tissues (data not shown). The null mice reproduced in numbers similar to their wild-type counterparts, with no change in litter size or sexual make-up. Body weight was monitored for a period of 6 months from birth, and it was found that there was no significant difference in body weight between null and wild-type animals of either sex (data not shown). However, at 6 months of age, a number of male null mice weighed in excess of 70 g, whereas none of age-matched male wild-type animals achieved this weight.
Hepatic RNA samples from GstP1/P2(−/−), GstP1/P2(+/−), and GstP1/P2(+/+) mice were probed with the GstP1 cDNA (Fig. 2); the wild-type mice demonstrated the previously reported sexual dimorphism in GstP expression, which also was evident in the heterozygote mice, but with lower levels of expression consistent with the loss of one GstP allele. GSTP1/P2 mRNA was completely absent in the null mice of both sexes. These findings were confirmed by immunoblotting with hepatic cytosolic samples (Fig. 2), and by measurement of activity of these cytosolic samples toward ethacrynic acid, a marker substrate for GstP (Fig. 2C). The absence of any ethacrynic acid activity in the null animals demonstrates the specificity of this substrate for these enzymes. Interestingly, when activity toward CDNB, a general GST substrate, was determined, although heterozygote mice displayed a slight drop in activity, little change was evident between the null and wild-type mice.
Figure 2.
Characterization of GstP1/P2(−/−), GstP1/P2(+/−), and GstP1/P2(+/+) mice. Samples were from male (M) and female (F) wild-type (+/+), heterozygote (+/−), and null (−/−) mice. (A) Northern analysis of hepatic RNA (10 μg per lane), using a cDNA representing full-length GstP1. Sample integrity was confirmed by staining of 16S and 28S rRNA in the gel with ethidium bromide before transfer to nitrocellulose, and equivalence of loading was confirmed by using a cDNA for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Western blot analysis of hepatic cytosol (10 μg protein per lane), using a polyclonal antiserum raised against the mouse GstP1–2 protein and ECL detection (Amersham). Equivalence of loading was confirmed by Coomassie blue staining of a duplicate SDS/PAGE gel. (C) Enzymatic activity of hepatic cytosol toward ethacrynic acid and CDNB was measured spectrophotometrically. Data represent mean of five animals ± SEM. (D) Western blot analysis of hepatic cytosol (10 μg protein per lane), using antisera toward GST from alpha and mu families (38, 39), and ECL detection (Amersham). Equivalence of loading was confirmed by Coomassie blue staining of a duplicate SDS/PAGE gel.
The expression of GST from other gene families (Fig. 2D) was not changed as a result of GstP1/P2 deletion. Furthermore, no change in the expression of various P450 isoenzymes, i.e., CYP1A2, CYP2E1, and CYP3A4, was observed (data not shown).
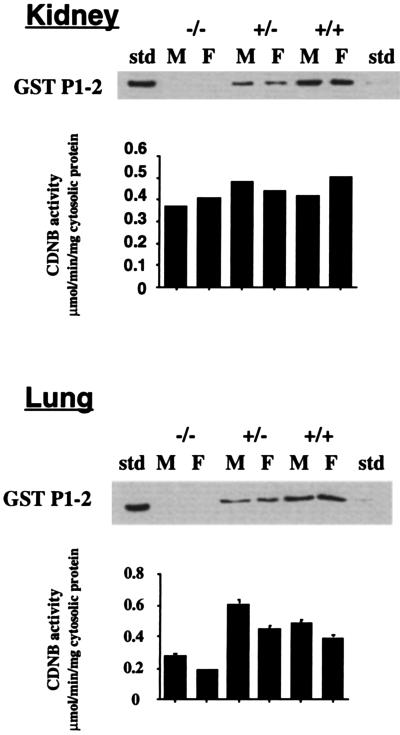
Cytosolic samples from kidney and lung also were analyzed for GstP expression (Fig. 3); In both tissues, GstP1 was decreased in the heterozygotes, and completely absent in the null mice. In the kidney, activity toward CDNB was similar in all mouse lines. However, in the lung, there was a significant decrease in the metabolism of CDNB in null mice of both sexes (Fig. 3), indicating that GstP1 plays a major role in GST activity in this tissue. GstP has been reported to be the major isoenzyme found in both human and rodent skin (42); neither the GstP protein, nor associated ethacrynic acid activity, could be detected in the skin of GstP1/P2(−/−) mice (not shown).
Figure 3.
GstP expression in lung and kidney. Samples were from male (M) and female (F) wild-type (+/+), heterozygote (+/−), and null (−/−) mice. Western blot analysis of kidney and lung cytosolic samples (10 μg protein per lane), using a polyclonal antiserum raised against the mouse GstP1–2 protein (36) and ECL detection (Amersham). Equivalence of loading was confirmed by Coomassie blue staining of a duplicate SDS/PAGE gel. Activity of kidney and lung cytosolic samples toward CDNB was measured spectrophotometrically and is shown in the corresponding bar charts. Values are expressed as mean ± SEM from five determinations
Total GSH content of hepatic cytosolic samples was determined (Fig. 4), and no difference was found according to sex or genotype. In addition, no change in the levels of expression of GSH synthetase or the light chain of γ-glutamylcysteine synthetase was found (Fig. 4).
Figure 4.
Expression of GSH-dependent enzymes in GstP null mice. Samples were from male (M) and female (F) wild-type (+/+), heterozygote (+/−), and null (−/−) mice. Western blot analysis of hepatic cytosolic samples (10 μg protein per lane), with antisera to GSH synthetase and γ-glutamylcysteine synthetase (light chain). Total GSH content was determined in hepatic cytosol as detailed in Materials and Methods. Values are expressed as mean ± SEM from five determinations.
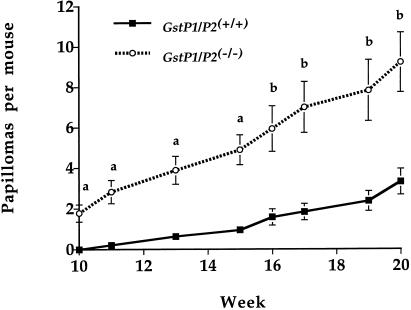
Modulation of drug-metabolizing enzymes in vivo can have a profound effect on the carcinogenic effects of chemicals (43). In certain cases, such chemoprotection has been ascribed to alterations in the expression of GSTs (44–46). However, chemoprotectors often have multiple effects on patterns of gene expression and protection has not been attributed to one individual enzyme. The GstP null mice therefore allowed us to test the hypothesis that this gene family plays a role in carcinogenesis. A two-stage carcinogenesis protocol using the PAH DMBA was used. Animals were treated topically with a single application of DMBA, and thereafter twice weekly with the tumor-promoting agent TPA for 20 weeks. Papillomas first started to form in the GstP null mice at 10 weeks, at which point none were observed in control animals. A steady increase in papilloma formation then was observed in both groups; however, at all points during the test period tumor incidence (Fig. 5) was markedly higher in the GstP1/P2(−/−) mice, being 10-fold higher at week 11, and 3.5-fold greater at week 20 (GstP1/P2(−/−), 18 animals, 179 papilloma, mean = 9.94, GstP1/P2(+/+), 19 animals, 55 papillomas, mean = 2.89, P < 0.001). Shortly after this time point, the tumor burden in the GstP1/P2(−/−) mice became so great that is was necessary to cull the majority of animals in this group. Interestingly, 40 weeks after initial treatment, whereas almost all of the GstP1/P2(−/−) mice had been culled, more than half of the wild-type mice in the control group were still alive. This further exemplifies the marked difference in susceptibility between the two groups.
Figure 5.
Tumor incidence of papillomas in mice treated with DMBA and TPA. Susceptibility of the mice to the tumorigenic effects of DMBA was determined by using the initiation-promotion protocol outlined in Material and Methods. After treatment, GstP1/P2(−/−) and GstP1/P2(+/+) mice were scored for papilloma numbers on a weekly basis, and statistical analysis was carried out by using the Statview software package (Abacus Concepts, Berkeley, CA). a = P < 0.0001, b = P < 0.001.
DISCUSSION
GSTs appear to play a major role in determining the sensitivity of cells to toxic and carcinogenic agents (3, 44, 45). GSTP is of potentially significant importance in this regard in view of its wide tissue distribution. In addition, GSTP is often overexpressed in human tumors and in cells made resistant to carcinogens and chemotherapeutic drugs, although its direct role in carcinogenesis or in generating the resistance phenotype remains obscure.
The deletion of GstP from the mouse has allowed us to evaluate the in vivo functions of this specific family of proteins. GstP is not essential for life, nor does it appear to support any critical physiological function. The observation that some of the null mice have a significantly increased body weight is interesting but at present unexplained. The finding that GST activity toward CDNB is markedly reduced in lung tissue confirms that it is the major enzyme in this organ (47). Interestingly, however, significant pulmonary CDNB activity remained in the null animals, indicating that other GST isoenzymes are also important in the lung. In both liver and kidney GstP does not play a major role in CDNB metabolism; however, in all tissues studied, including the skin, activity toward ethacrynic acid was completely absent. These data demonstrate the specificity of this substrate for GstP.
There were no compensatory changes in the level of expression of the other classes of GST in mouse liver as a result of deletion of GstP, enzymes in the α- and μ-classes remaining unchanged in nulls and heterozygotes. Similarly, there is no discernible change in GSH content in hepatic cytosol between wild-type, heterozygote, and null mice (Fig. 4), nor in key enzymes responsible for GSH homeostasis, γ-glutamylcysteine synthetase, or GSH synthetase. GstP therefore does not appear to be involved in the maintenance of GSH levels, at least in the unstressed animal.
GstP has a broad substrate specificity but of particular interest is its capacity to detoxify the carcinogenic products of PAH metabolism (48). For this reason, we investigated whether susceptibility to PAH-induced carcinogenesis is altered in GstP null mice. GstP1/P2(−/−) and GstP1/P2(+/+) mice were used in a two-stage skin carcinogenesis protocol (41). In this paradigm, tumor development is initiated by carcinogen treatment, followed by repeated application of a tumor promotor. This process leads to persistent general hyperplasia and the formation of multiple benign tumors or papillomas. Approximately 5–10% of papillomas progress to malignancy, although this figure may be increased by treatment with mutagens.
Female GstP1/P2(−/−) and GstP1/P2(+/+) mice were treated topically with a single application of DMBA, and thereafter twice weekly with TPA for 20 weeks. At all time points of the study, there was a highly significant (P < 0.001) increase in papilloma formation in the GstP null mice relative to the controls. This difference ranged from greater than 10-fold when papillomas first formed to 3.5-fold at 20 weeks. At the present time we do not know whether the difference in tumor incidence is caused by alterations in the mutagenic effects of the initiating agent or to the promotional effects of TPA. This is a current theme of ongoing studies, but based on our present knowledge of this enzyme the former possibility would appear most likely. GSTP1 is the enzyme with the highest activity in the detoxification of the ultimate carcinogenic metabolite of benzo[a]pyrene,(+)-anti-7,8-dihydroxy-9,10-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Hu et al., (48) reported that GstP1 was more effective in the detoxification of this compound in mouse liver and forestomach than all other GSTs combined.
The data presented in this paper, although only pertaining to benign tumors, provide unequivocal evidence that GstP can play a critical role in PAH-induced skin tumorigenesis, and further demonstrates that modulation of a single drug metabolizing enzyme can have a profound effect on susceptibility to carcinogens. It therefore would be predicted that individuality in the levels of GstP may effect cellular sensitivity to the carcinogenic effects of GstP substrates.
Exposure to PAH is thought to play a pivotal role in human cancers associated with cigarette smoking, such as tumors of the lung and bladder. Indeed, this enzyme is the major GST found in human lung and bladder tissue (47, 49). GSTP1 is polymorphic in humans, with allelic variants demonstrating differences in their catalytic activities toward a range of substrates including important pulmonary carcinogens such as (+)-anti-7,8-dihydroxy-9,10-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene [(+)-anti-BPDE] (50, 51). It also has been reported that the architecture of the active site of human GSTP1 can account for the enantioselectivity displayed by the enzyme in its detoxification of (+)-anti-BPDE and related isomers (52). The authors suggest that the disparate enzyme activities of the polymorphic variants of GSTP1 may at least partly account for interindividual variation in susceptibility to carcinogens such as (+)-anti-BPDE. In support of this possibility, Ryberg et al., reported that the level of PAH-DNA adducts in smokers lungs is higher in individuals carrying one of these allelic variants (53). It also has been reported that there is an altered distribution of GSTP1 alleles in both bladder and lung cancer (51, 53). The finding that GstP can profoundly alter susceptibility to carcinogenesis in the mouse indicates that individual variability in the expression of this gene also may be an important factor in cancer susceptibility in humans.
Acknowledgments
We thank Del Watling and Tracey Crafton at the Imperial Cancer Research Fund Clare Hall Laboratories, Louise Anderson and Vanessa McGilliard at the Centre for Genome Research, and Sheila Bryson at The Beatson Institute, for their animal handling skills. We are also grateful to Prof. John Hayes for antisera to various GST isoenzymes, and to Dr. Lesley McLellan for help and advice in GSH measurements. Dr. Brian McStay is thanked for critical reading of this manuscript. The glyceraldehyde phosphate dehydrogenase probe was a gift from Dr. Rex Tyrell. Prof. Rick Lathe of the Centre for Genome Research is also acknowledged.
ABBREVIATIONS
- GST
glutathione S-transferase
- CDNB
1-chloro-2,4-dinitrobenzene
- DMBA
7,12-dimethylbenz anthracene
- PAH
polycyclic aromatic hydrocarbon
- GSH
glutathione
- TPA
12-O-tetradecanoylphorbol-13-acetate
References
- 1.Rushmore T H, Pickett C B. J Biol Chem. 1993;268:11475–11478. [PubMed] [Google Scholar]
- 2.Hayes J D, Strange R C. Free Radical Res. 1995;22:193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 3.Hayes J D, Pulford D J. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 4.Farber E. Cancer J Biochem Cell Biol. 1984;62:486–494. doi: 10.1139/o84-066. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Kitahara A, Satoh K, Ishikawa T, Tatematsu M, Ito N. Gann. 1984;75:199–202. [PubMed] [Google Scholar]
- 6.Satoh K, Kitahara A, Soma Y, Inaba Y, Hatayama I, Sato K. Proc Natl Acad Sci USA. 1985;82:3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K. Adv Cancer Res. 1989;52:205–255. doi: 10.1016/s0065-230x(08)60214-6. [DOI] [PubMed] [Google Scholar]
- 8.Sawaki M, Enomoto K, Takahashi H, Nakajima Y, Mori M. Carcinogenesis. 1990;11:1857–1861. doi: 10.1093/carcin/11.10.1857. [DOI] [PubMed] [Google Scholar]
- 9.Batist G, Tulpule A, Sinha B K, Katki A G, Myers C E, Cowan K H. J Biol Chem. 1986;261:15544–15549. [PubMed] [Google Scholar]
- 10.Cowan K H, Batist G, Tulpule A, Sinha B K, Myers C E. Proc Natl Acad Sci USA. 1986;83:9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black S M, Wolf C R. Pharmacol Ther. 1991;51:139–154. doi: 10.1016/0163-7258(91)90044-m. [DOI] [PubMed] [Google Scholar]
- 12.Wolf C R, Wareing C J, Black S M, Hayes J D. In: Glutathione S-Transferases In Resistance to Chemotherapeutic Drugs. Hayes J D, Pickett C B, Mantle T J, editors. London: Taylor & Francis; 1990. pp. 296–307. [Google Scholar]
- 13.Tew K D. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 14.O’Brien M L, Tew K D. Eur J Cancer. 1996;32A:967–978. doi: 10.1016/0959-8049(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 15.Schipper D, Wagenmans M, Wagener D, Peters W. Int J Oncol. 1997;10:1261–1264. doi: 10.3892/ijo.10.6.1261. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Ishida T, Sugio K, Maehara Y, Sugimachi K. Respiration. 1995;62:223–227. doi: 10.1159/000196451. [DOI] [PubMed] [Google Scholar]
- 17.Green J A, Robertson L J, Clark A H. Br J Cancer. 1993;68:235–239. doi: 10.1038/bjc.1993.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katagiri A, Tomita Y, Nishiyama T, Kimura M, Sato S. Br J Cancer. 1993;68:125–129. doi: 10.1038/bjc.1993.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S V, Xu B H, Gupta V, Emerson E O, Zaren H A, Jani J P. Cancer Lett. 1995;95:49–56. doi: 10.1016/0304-3835(95)03864-s. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Xiao Y, Priddy R. J Oral Pathol Med. 1994;23:75–79. doi: 10.1111/j.1600-0714.1994.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 21.Grignon D J, Abdel Malak M, Mertens W C, Sakr W A, Shepherd R R. Mod Pathol. 1994;7:186–189. [PubMed] [Google Scholar]
- 22.Hamada S, Kamada M, Furumoto H, Hirao T, Aono T. Gynecol Oncol. 1994;52:313–319. doi: 10.1006/gyno.1994.1055. [DOI] [PubMed] [Google Scholar]
- 23.Lee W H, Morton R A, Epstein J I, Brooks J D, Campbell P A, Bova G S, Hsieh W S, Isaacs W B, Nelson W G. Proc Natl Acad Sci USA. 1994;91:11733–11737. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cookson M S, Reuter V E, Linkov I, Fair W R. J Urol. 1997;157:673–676. [PubMed] [Google Scholar]
- 25.Howie A F, Forrester L M, Glancey M J, Schlager J J, Powis G, Beckett G J, Hayes J D, Wolf C R. Carcinogenesis. 1990;11:451–458. doi: 10.1093/carcin/11.3.451. [DOI] [PubMed] [Google Scholar]
- 26.Lewis A D, Forrester L M, Hayes J D, Wareing C J, Carmichael J, Harris A L, Mooghen M, Wolf C R. Br J Cancer. 1989;60:327–331. doi: 10.1038/bjc.1989.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moffat G J, McLaren A W, Wolf C R. J Biol Chem. 1994;269:16397–16402. [PubMed] [Google Scholar]
- 28.Xia C, Hu J, Ketterer B, Taylor J B. Biochem J. 1996;313:155–161. doi: 10.1042/bj3130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moffat G J, McLaren A W, Wolf C R. Biochem J. 1997;324:91–95. doi: 10.1042/bj3240091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bammler T K, Smith C A, Wolf C R. Biochem J. 1994;298:385–390. doi: 10.1042/bj2980385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bammler T K, Driessen H, Finnstrom N, Wolf C R. Biochemistry. 1995;34:9000–9008. doi: 10.1021/bi00028a008. [DOI] [PubMed] [Google Scholar]
- 32.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meehan R R, Barlow D P, Hill R E, Hogan B L M, Hastie N D. EMBO J. 1984;3:1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chomczynski P. Anal Biochem. 1992;201:134–139. doi: 10.1016/0003-2697(92)90185-a. [DOI] [PubMed] [Google Scholar]
- 36.McLellan L I, Hayes J D. Biochem J. 1987;245:399–406. doi: 10.1042/bj2450399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson C J, Wolf C R. In: Immunodetection of Proteins by Immunoblotting. Manson M M, editor. Vol. 10. Totowa, NJ: Humana; 1992. pp. 221–234. [Google Scholar]
- 38.Hayes J D, Mantle T J. Biochem J. 1986;233:779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes J D, Coulthwaite R E, Stockman P K, Hussey A J, Mantle T J, Wolf C R. Arch Toxicol Suppl. 1987;10:136–146. doi: 10.1007/978-3-642-71617-1_11. [DOI] [PubMed] [Google Scholar]
- 40.Henderson C J, Scott A R, Yang C S, Wolf C R. Biochem J. 1990;266:675–681. doi: 10.1042/bj2660675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown K, Balmain A. Cancer Metastasis Rev. 1995;14:113–124. doi: 10.1007/BF00665795. [DOI] [PubMed] [Google Scholar]
- 42.Raza H, Awasthi Y C, Zaim M T, Eckert R L, Mukhtar H. J Invest Dermatol. 1991;96:463–467. doi: 10.1111/1523-1747.ep12470150. [DOI] [PubMed] [Google Scholar]
- 43.Prochaska H J, Talalay P. Cancer Res. 1988;48:4776–4782. [PubMed] [Google Scholar]
- 44.Talalay P, Fahey J W, Holtzclaw W D, Prestera T, Zhang Y. Toxicol Lett. 1995;82–83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 45.Kensler T W, Davidson N E, Groopman J D, Roebuck B D, Prochaska H J, Talalay P. Basic Life Sci. 1993;61:127–136. doi: 10.1007/978-1-4615-2984-2_12. [DOI] [PubMed] [Google Scholar]
- 46.Buetler T M, Gallagher E P, Wang C, Stahl D L, Hayes J D, Eaton D L. Toxicol Appl Pharmacol. 1995;135:45–57. doi: 10.1006/taap.1995.1207. [DOI] [PubMed] [Google Scholar]
- 47.Carmichael J, Forrester L M, Lewis A D, Hayes J D, Hayes P C, Wolf C R. Carcinogenesis. 1988;9:1617–1621. doi: 10.1093/carcin/9.9.1617. [DOI] [PubMed] [Google Scholar]
- 48.Hu X, Benson P, Srivastava S, Xia H, Bleicher R, Zaren H, Awasthi S, Awasthi Y, Singh S. Int J Cancer. 1997;73:897–902. doi: 10.1002/(sici)1097-0215(19971210)73:6<897::aid-ijc23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Wolf C R, Lewis A D, Carmichael J, Adams D J, Allan S G, Ansell D J. Biochem Soc Trans. 1987;15:728–730. doi: 10.1042/bst0150728. [DOI] [PubMed] [Google Scholar]
- 50.Ali-Osman F, Akande O, Antoun G, Mao J X, Buolamwini J. J Biol Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 51.Harries L W, Stubbins M J, Forman D, Howard G C, Wolf C R. Carcinogenesis. 1997;18:641–644. doi: 10.1093/carcin/18.4.641. [DOI] [PubMed] [Google Scholar]
- 52.Hu X, O’Donnell R, Srivastava S K, Xia H, Zimniak P, Nanduri B, Bleicher R J, Awasthi S, Awasthi Y C, Ji X, Singh S V. Biochem Biophys Res Commun. 1997;235:424–428. doi: 10.1006/bbrc.1997.6777. [DOI] [PubMed] [Google Scholar]
- 53.Ryberg D, Skaug V, Hewer A, Phillips D H, Harries L W, Wolf C R, Ogreid D, Ulvik A, Vu P, Haugen A. Carcinogenesis. 1997;18:1285–1289. doi: 10.1093/carcin/18.7.1285. [DOI] [PubMed] [Google Scholar]