Abstract
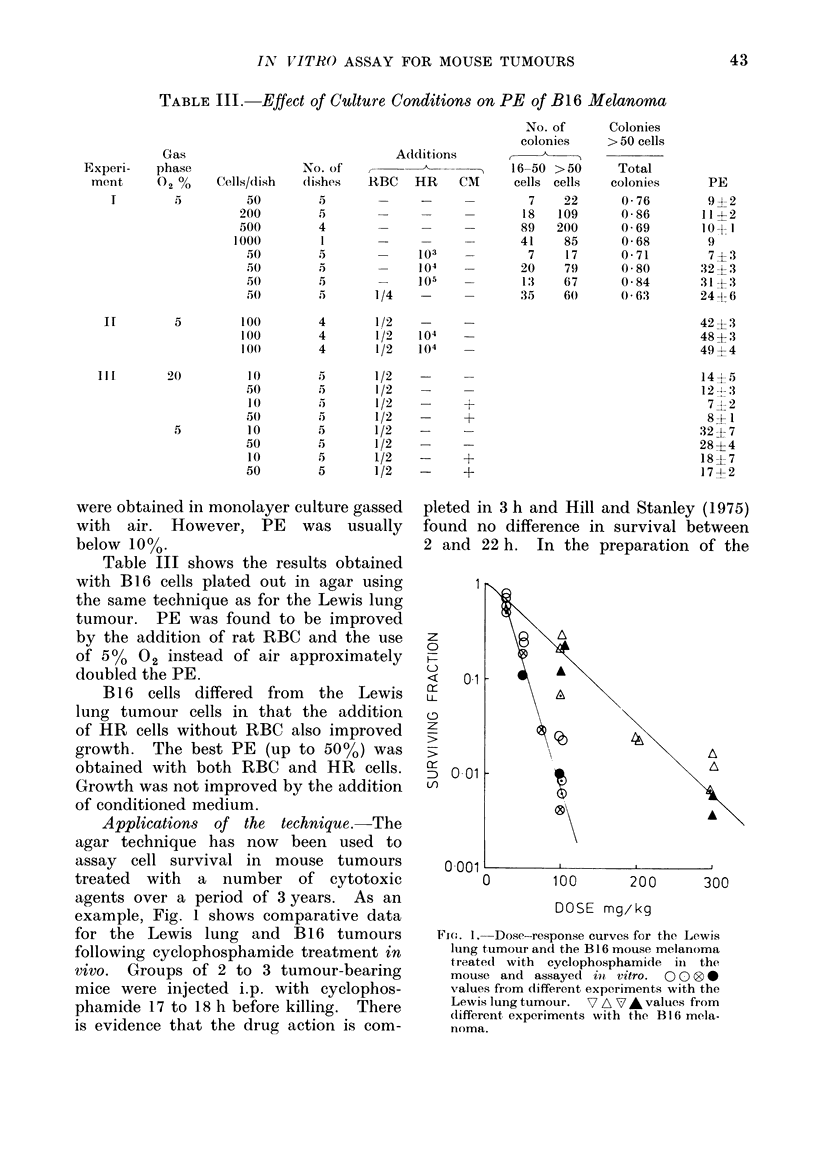
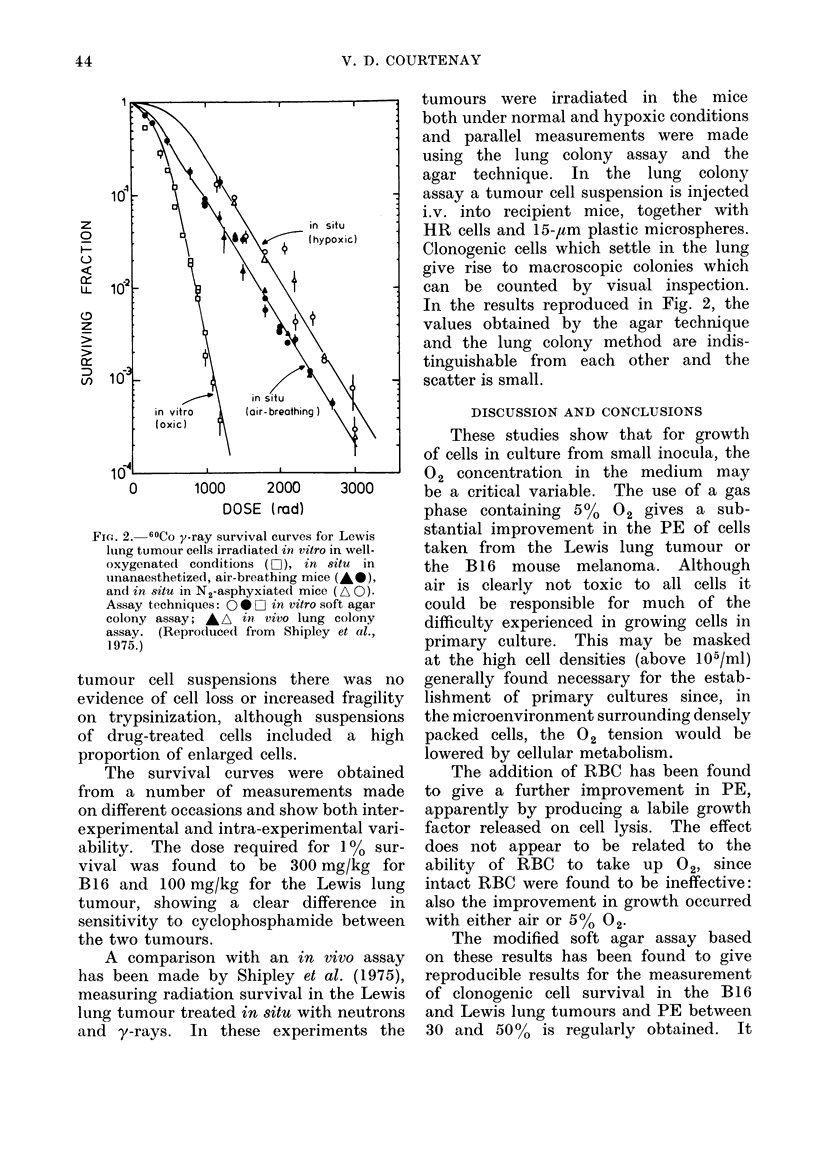
A soft agar colony assay has been developed for the B16 mouse melanoma and the Lewis lung tumour. The special features of the technique are the use of a gas phase with 5% O2 instead of air and the addition of rat red blood cells. Single cell suspensions are prepared by trypsinization from the solid tumour and the cells are plated out in 0-3% agar over a layer of 0-5% agar in 30-mm Petri dishes. After 8 to 15 days' incubation in 5% O2, colonies of more than 50 cells are produced. Plating efficiencies of between 30 and 50% are usually obtained. The addition of up to 10(4) heavily irradiated tumour cells gives some further improvement in plating efficiency for the B16 melanoma but not for the Lewis lung tumour. Applications of the technique to measure cell survival in the two tumours after treatment with cytotoxic drugs and radiation are reported. The scatter of experimental points is relatively small, and in comparative experiments good agreement has been obtained with results using in vivo assay techniques.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Telfer P. A., Fry P. The effect of erythrocytes on mouse bone marrow colony development in vitro. Blood. 1971 Sep;38(3):353–359. [PubMed] [Google Scholar]
- Hill R. P., Stanley J. A. The response of hypoxic B16 melanoma cells to in vivo treatment with chemotherapeutic agents. Cancer Res. 1975 May;35(5):1147–1153. [PubMed] [Google Scholar]
- Ichikawa Y., Paran M., Sachs L. The stimulation of tumor cell growth by a substance produced by normal and tumor cells. J Cell Physiol. 1969 Feb;73(1):43–47. doi: 10.1002/jcp.1040730107. [DOI] [PubMed] [Google Scholar]
- JAMIESON D., VANDENBRENK H. A. EFFECT OF ELECTRODE DIMENSIONS ON TISSUE PO-2 MEASUREMENT IN VIVO. Nature. 1964 Mar 21;201:1227–1228. doi: 10.1038/2011227a0. [DOI] [PubMed] [Google Scholar]
- OSGOOD E. E., KRIPPAEHNE M. L. The gradient tissue culture method. Exp Cell Res. 1955 Aug;9(1):116–127. doi: 10.1016/0014-4827(55)90165-8. [DOI] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Shipley W. U., Stanley J. A., Courtenay V. D., Field S. B. Repair of radiation damage in Lewis lung carcinoma cells following in situ treatment with fast neutrons and gamma-rays. Cancer Res. 1975 Apr;35(4):932–938. [PubMed] [Google Scholar]
- Steel G. G., Adams K. Stem-cell survival and tumor control in the Lewis lung carcinoma. Cancer Res. 1975 Jun;35(6):1530–1535. [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]


