Abstract
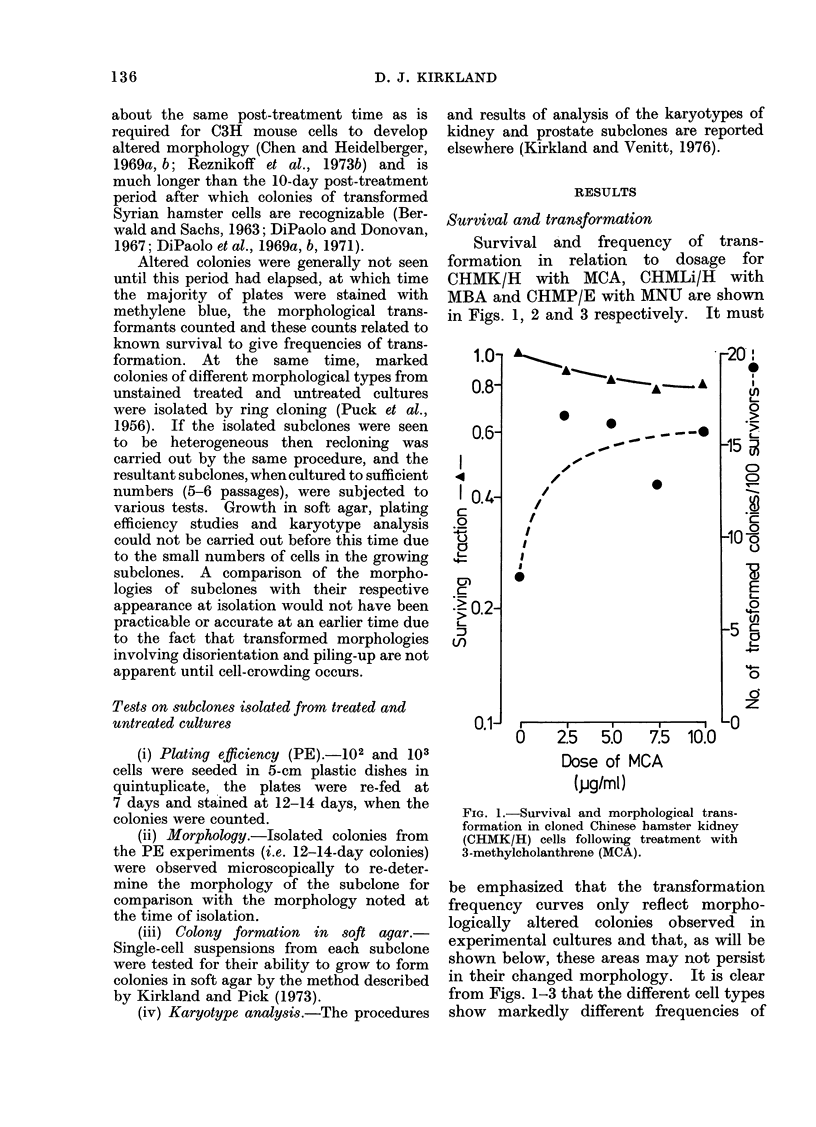
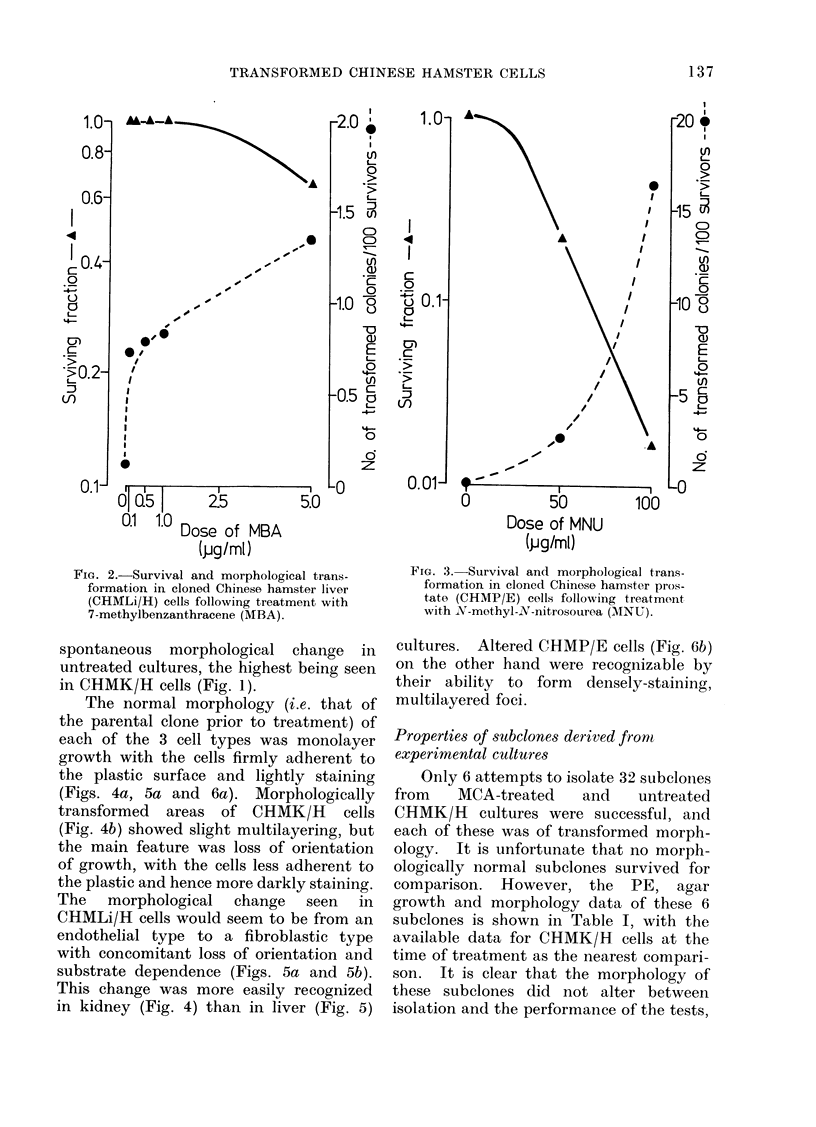
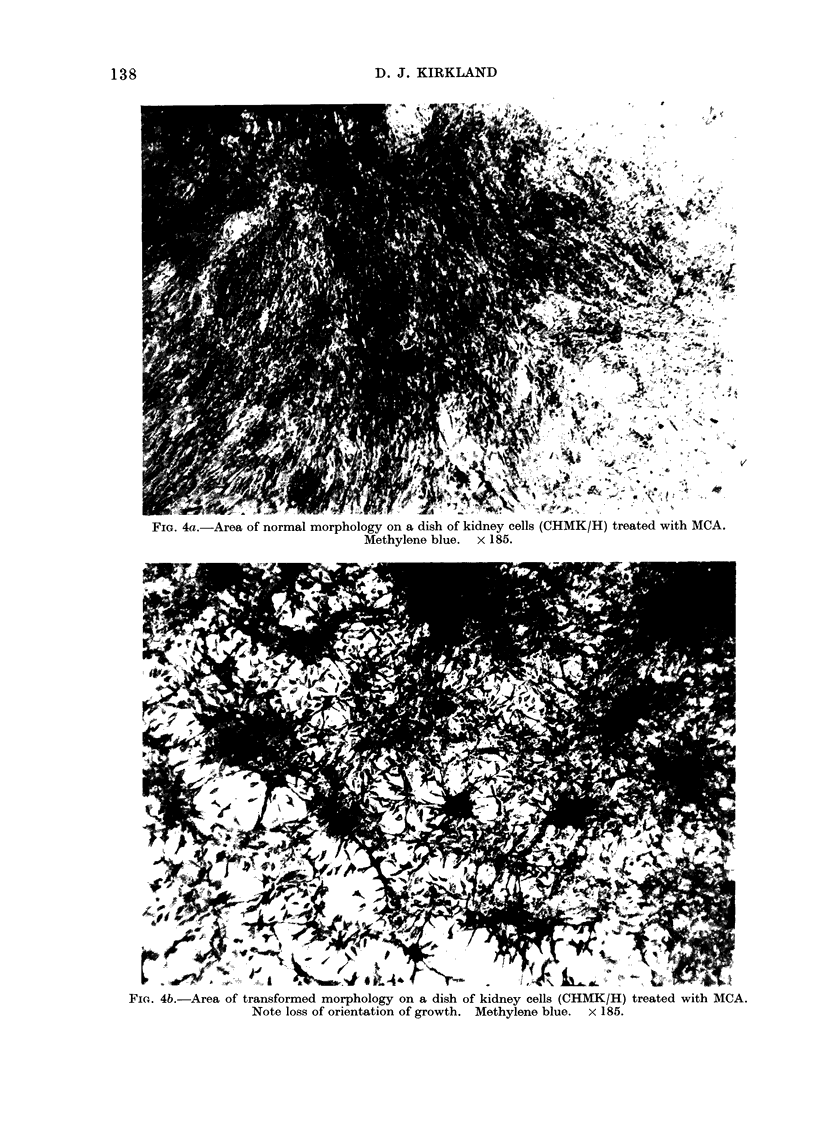
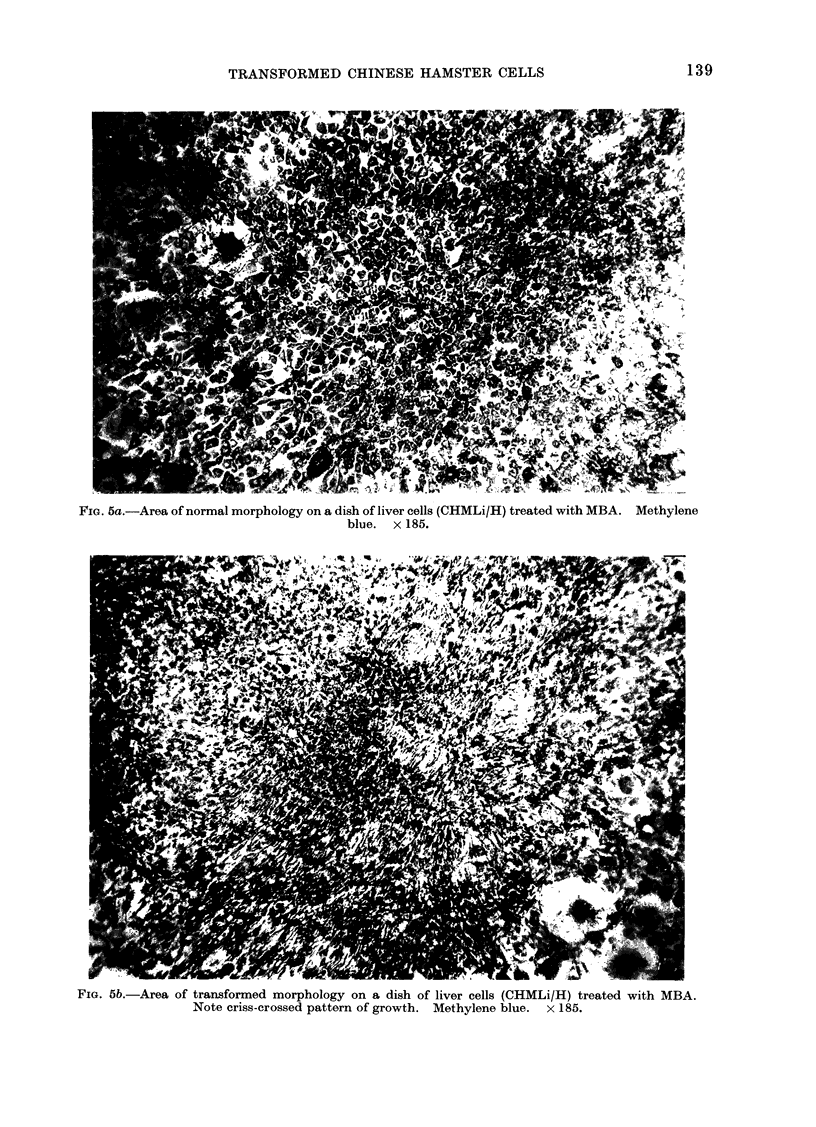
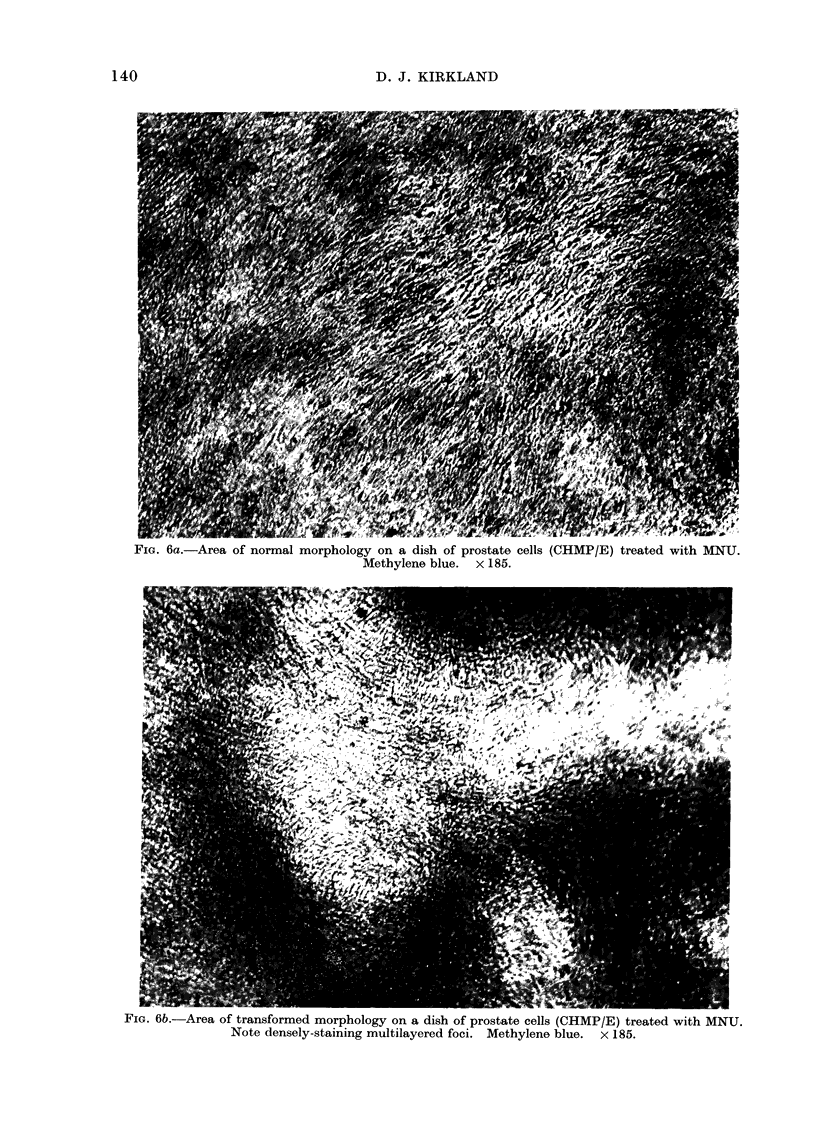
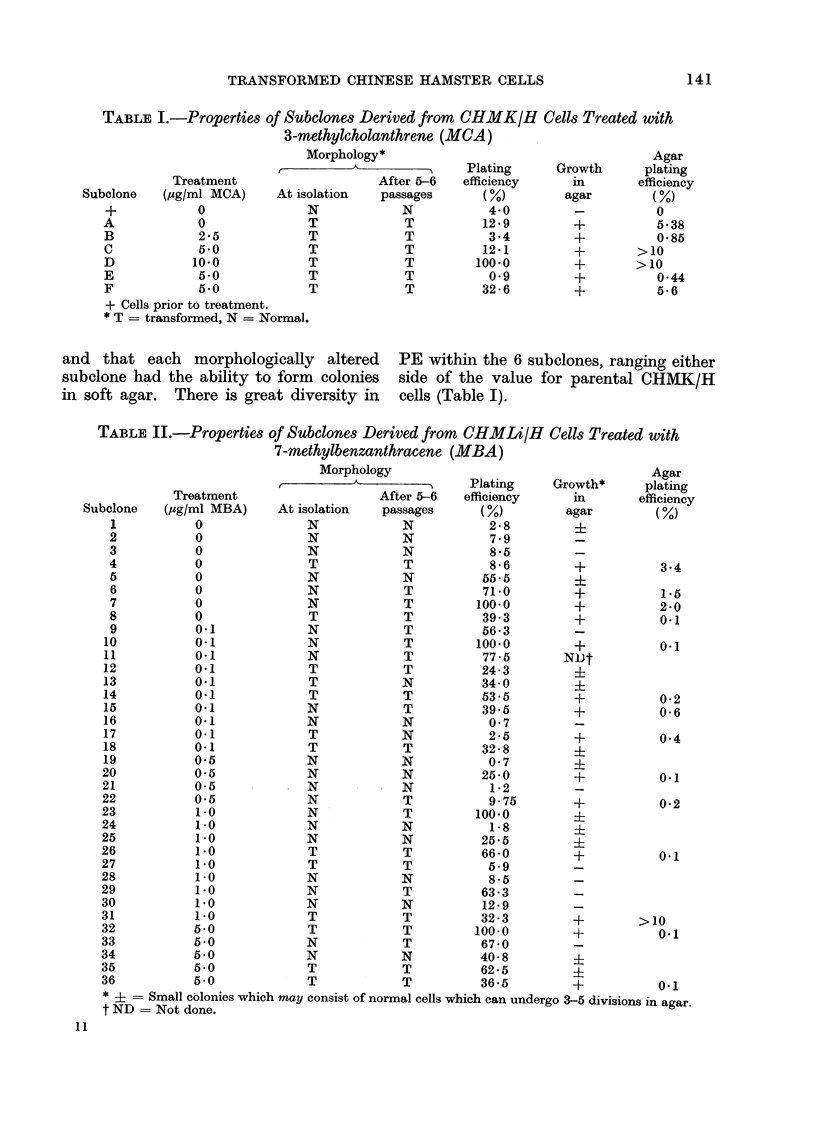
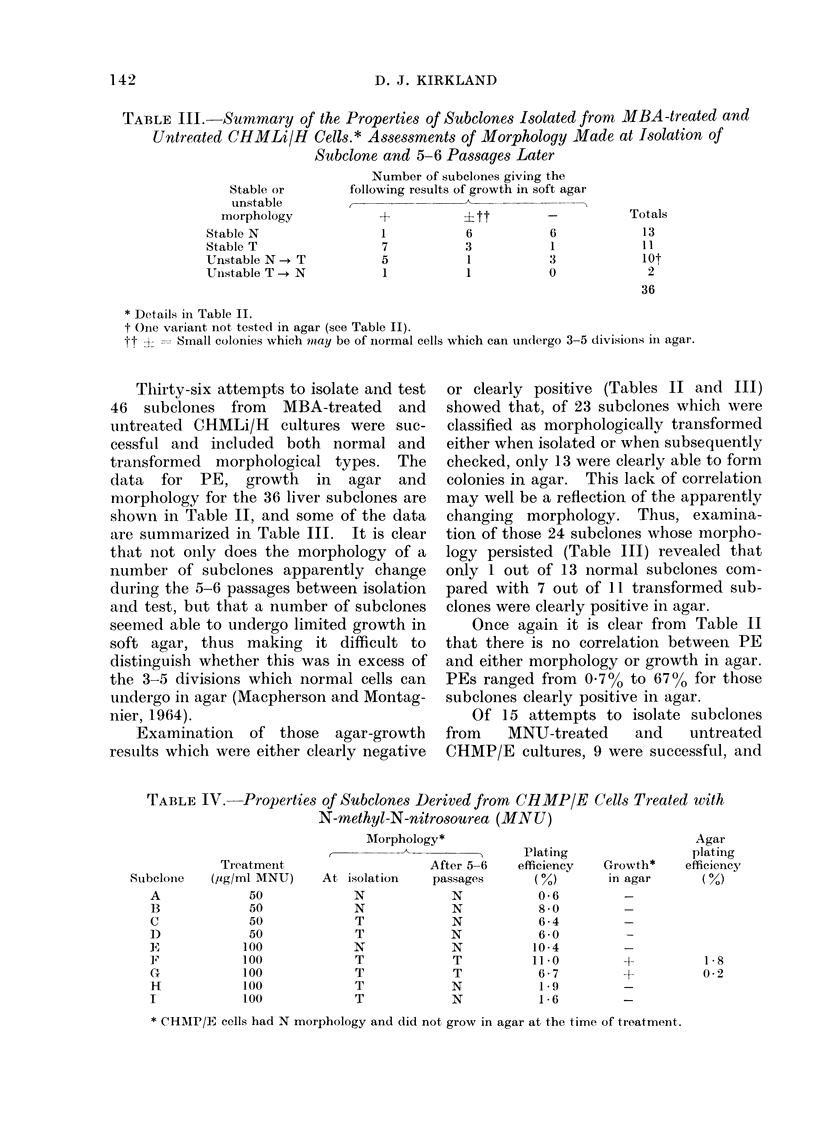
Fifty-one subclones from carcinogen-treated cells of 3 tissues (kidney, liver and prostate) of the male Chinese hamster have been studied to determine the relationships of 3 criteria of in vitro transformation: morphological change, increased plating efficiency and growth in soft agar. There was no correlation between increased plating efficiency and the other 2 parameters. Morphological change was not always easily recognisable, particularly in cells derived from liver, and was not always a stable feature of any given subclone. This may be due to the technique of isolation used (ring cloning) or may be due to chemically-treated cells requiring long periods of culturing before attaining a stable phenotype. When a stable morphological appearance was achieved, there was good correlation between transformed morphology and colony formation in soft agar. The problems of scoring morphological change as an assessment of malignant transformation, and the importance of spontaneous morphological changes are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERWALD Y., SACHS L. IN VITRO CELL TRANSFORMATION WITH CHEMICAL CARCINOGENS. Nature. 1963 Dec 21;200:1182–1184. doi: 10.1038/2001182a0. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Heidelberger C. In vitro malignant transformation of cells derid from mouse prostate in the presence of 3-methylcholanthrene. J Natl Cancer Inst. 1969 Jun;42(6):915–925. [PubMed] [Google Scholar]
- Chen T. T., Heidelberger C. Quantitative studies on the malignant transformation of mouse prostate cells by carcinogenic hydrocarbons in vitro. Int J Cancer. 1969 Mar 15;4(2):166–178. doi: 10.1002/ijc.2910040207. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P. J., Nelson R. L. In vitro transformation of hamster cells by polycyclic hydrocarbons: factors influencing the number of cells transformed. Nat New Biol. 1971 Apr 21;230(16):240–242. doi: 10.1038/newbio230240a0. [DOI] [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P., Nelson R. Quantitative studies of in vitro transformation by chemical carcinogens. J Natl Cancer Inst. 1969 May;42(5):867–874. [PubMed] [Google Scholar]
- DiPaolo J. A., Nelson R. L., Donovan P. J. Sarcoma-producing cell lines derived from clones transformed in vitro by benzo[a]pyrene. Science. 1969 Aug 29;165(3896):917–918. doi: 10.1126/science.165.3896.917. [DOI] [PubMed] [Google Scholar]
- Dipaolo J. A., Donovan P. J. Properties of Syrian hamster cells transformed in the presence of carcinogenic hydrocarbons. Exp Cell Res. 1967 Nov;48(2):361–377. doi: 10.1016/0014-4827(67)90361-8. [DOI] [PubMed] [Google Scholar]
- Evans C. H., DiPaolo J. A. Neoplastic transformation of guinea pig fetal cells in culture induced by chemical carcinogens. Cancer Res. 1975 Apr;35(4):1035–1044. [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Oliver J. Early enhancement of plating efficiency of primary mouse embryo cells by the carcinogen methylnitrosourea. Cancer Res. 1972 Dec;32(12):2747–2752. [PubMed] [Google Scholar]
- Kirkland D. J., Armstrong C., Harris R. J. Spontaneous and chemically induced transformation of rat embryo cell cultures. Br J Cancer. 1975 Mar;31(3):329–337. doi: 10.1038/bjc.1975.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland D. J., Pick C. R. The histological appearance of tumours derived from rat embryo cells transformed in vitro spontaneously and after treatment with nitrosomethylurea. Br J Cancer. 1973 Nov;28(5):440–452. doi: 10.1038/bjc.1973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland D. J., Venitt S. Chemical transformation of Chinese hamster cells: II. Appearance of marker chromosomes in transformed cells. Br J Cancer. 1976 Aug;34(2):145–152. doi: 10.1038/bjc.1976.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Sanford K. K., Handleman S. L., Fox C. H., Burris J. F., Hursey M. L., Mitchell J. T., Jackson J. L., Parshad R. Effects of chemical carcinogens on neoplastic transformation and morphology of cells in culture. J Natl Cancer Inst. 1974 Dec;53(6):1647–1659. [PubMed] [Google Scholar]








