Abstract
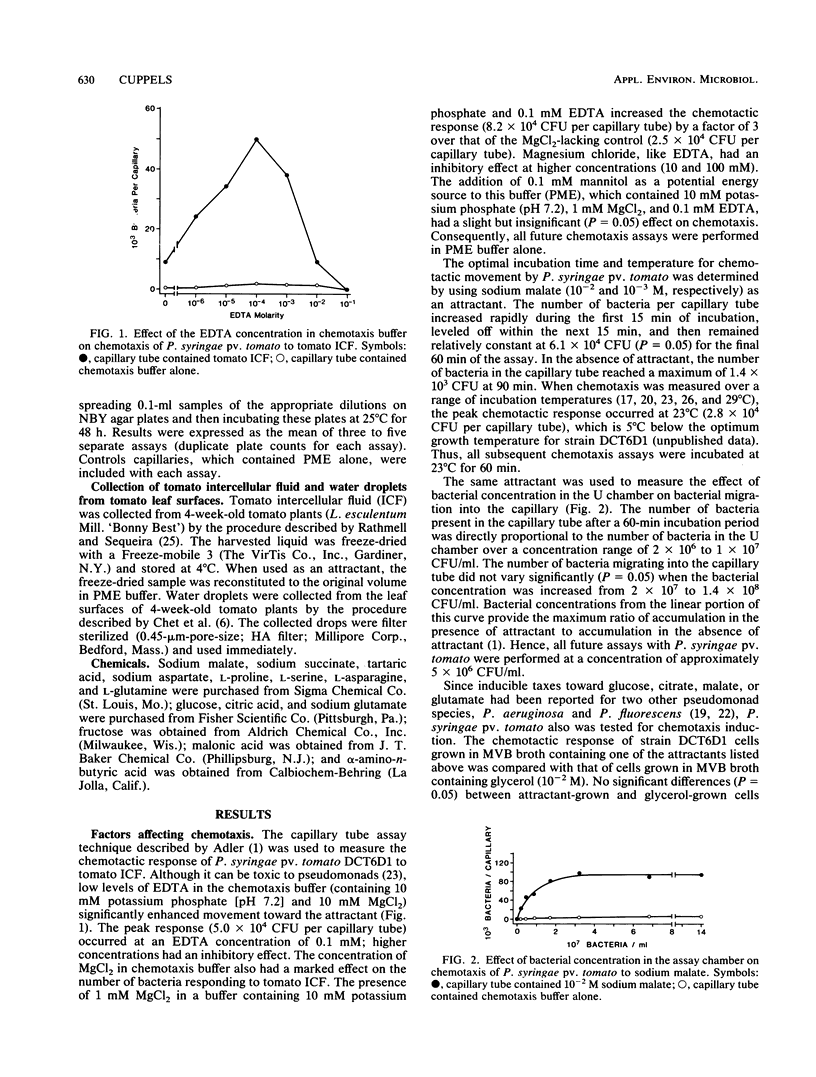
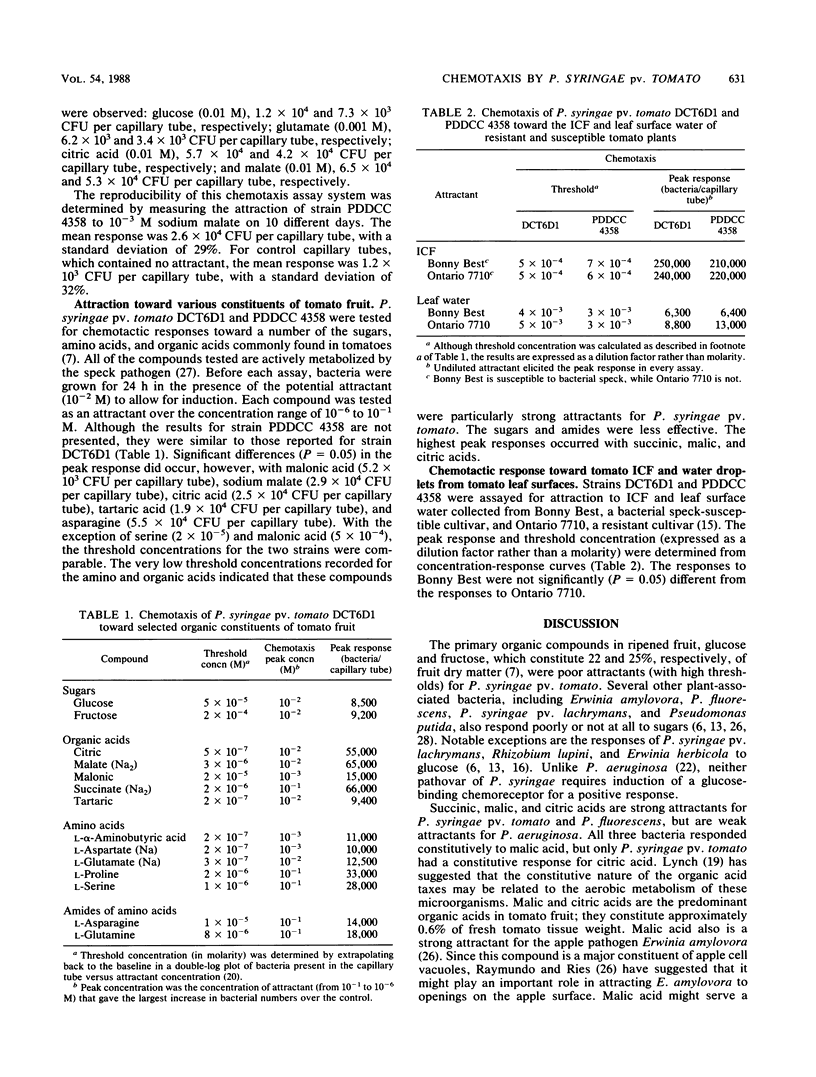
Optimal laboratory conditions for studying chemotaxis by Pseudomonas syringae pv. tomato were determined by using the Adler capillary tube assay. Although they are not an absolute requirement for chemotaxis, the presence of 0.1 mM EDTA and 1 mM MgCl2 in the chemotaxis buffer (10 mM potassium phosphate [pH 7.2]) significantly enhanced the response to attractant. The addition of mannitol as an energy source had little effect. The optimal temperature for chemotaxis was 23°C, which is 5°C below the optimal growth temperature for this pathogen. The best response occurred when the bacteria were exposed to attractant for 60 min at a concentration of approximately 5 × 106 CFU/ml. P. syringae pv. tomato was strongly attracted to citric and malic acids, which are the predominant organic acids in tomato fruit. With the exception of asparagine, the major amino acids of tomatoes were weak to moderate attractants. Glucose and fructose, which account for approximately 47% of tomato dry matter, also elicited poor responses. In assays with tomato intercellular fluid and leaf surface water, the bacterial speck pathogen could not chemotactically distinguish between a resistant and a susceptible cultivar of tomato.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. N., Hobson G. E. The constituents of tomato fruit--the influence of environment, nutrition, and genotype. Crit Rev Food Sci Nutr. 1981;15(3):205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- Gallucci K. K., Paerl H. W. Pseudomonas aeruginosa Chemotaxis Associated with Blooms of N(2)-Fixing Blue-Green Algae (Cyanobacteria). Appl Environ Microbiol. 1983 Feb;45(2):557–562. doi: 10.1128/aem.45.2.557-562.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Rivelli M., Ornston L. N. Aromatic acids are chemoattractants for Pseudomonas putida. J Bacteriol. 1984 Nov;160(2):622–628. doi: 10.1128/jb.160.2.622-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- Lynch W. H. Effect of temperature on Pseudomonas fluorescens chemotaxis. J Bacteriol. 1980 Jul;143(1):338–342. doi: 10.1128/jb.143.1.338-342.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesibov R., Ordal G. W., Adler J. The range of attractant concentrations for bacterial chemotaxis and the threshold and size of response over this range. Weber law and related phenomena. J Gen Physiol. 1973 Aug;62(2):203–223. doi: 10.1085/jgp.62.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench T. T., Konetzka W. A. Chemotaxis in Pseudomonas aeruginosa. J Bacteriol. 1978 Jan;133(1):427–429. doi: 10.1128/jb.133.1.427-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton R. C., Montie T. C. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W. G., Sequeira L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974 Feb;53(2):317–318. doi: 10.1104/pp.53.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands D. C., Schroth M. N., Hildebrand D. C. Taxonomy of phytopathogenic pseudomonads. J Bacteriol. 1970 Jan;101(1):9–23. doi: 10.1128/jb.101.1.9-23.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


