Abstract
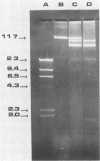
A psychrotrophic bacterium, originally isolated from a natural aquatic environment, was characterized and identified as Pseudomonas putida Q5 for use as a representative recipient for biodegradative genes from a mesophilic microorganism. The TOL plasmid pWWO of the mesophile P. putida PaW1 was successfully transferred by conjugation to the naturally isolated psychrotroph P. putida Q5, as shown by plasmid analysis by agarose gel electrophoresis. Expression of the genes encoded by the mesophilic TOL plasmid in the psychrotroph was shown by the fact that the transconjugant (designated P. putida Q5T) had the capacity to degrade and utilize toluate (1,000 mg/liter) as a sole source of carbon at temperatures as low as 0 degrees C. Comparison of growth rates over a wide temperature range (0 to 30 degrees C) indicated that the physiological activity of the transconjugant was not reduced and that the plasmid DNA from the mesophile and its encoded enzymes functioned effectively in the psychrotroph at temperatures well below those at which the mesophile could grow. The production and demonstrated functioning of P. putida Q5T illustrates the possibility of developing specific degradative capacities in bacteria which can readily function at low temperatures in chemically contaminated environments or in industrial wastewater treatment systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duggleby C. J., Bayley S. A., Worsey M. J., Williams P. A., Broda P. Molecular sizes and relationships of TOL plasmids in Pseudomonas. J Bacteriol. 1977 Jun;130(3):1274–1280. doi: 10.1128/jb.130.3.1274-1280.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni G. D., Inniss W. E. Thermally caused filament formation in the psychrophile Bacillus insolitus. Can J Microbiol. 1973 May;19(5):581–584. doi: 10.1139/m73-095. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Lehrbach P. R., Lurz R., Rueckert B., Bagdasarian M., Timmis K. N. Localization and functional analysis of transposon mutations in regulatory genes of the TOL catabolic pathway. J Bacteriol. 1983 May;154(2):676–685. doi: 10.1128/jb.154.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounot A. M., Novitsky T. J., Kushner D. J. Effects of temperature on the macromolecular composition and find structure of psychrophilic Arthrobacter species. Can J Microbiol. 1977 Mar;23(3):357–362. doi: 10.1139/m77-053. [DOI] [PubMed] [Google Scholar]
- Harayama S., Lehrbach P. R., Timmis K. N. Transposon mutagenesis analysis of meta-cleavage pathway operon genes of the TOL plasmid of Pseudomonas putida mt-2. J Bacteriol. 1984 Oct;160(1):251–255. doi: 10.1128/jb.160.1.251-255.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Leppik R. A., Rekik M., Mermod N., Lehrbach P. R., Reineke W., Timmis K. N. Gene order of the TOL catabolic plasmid upper pathway operon and oxidation of both toluene and benzyl alcohol by the xylA product. J Bacteriol. 1986 Aug;167(2):455–461. doi: 10.1128/jb.167.2.455-461.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inniss W. E. Interaction of temperature and psychrophilic microorganisms. Annu Rev Microbiol. 1975;29:445–465. doi: 10.1146/annurev.mi.29.100175.002305. [DOI] [PubMed] [Google Scholar]
- Inouye S., Ebina Y., Nakazawa A., Nakazawa T. Nucleotide sequence surrounding transcription initiation site of xylABC operon on TOL plasmid of Pseudomonas putida. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1688–1691. doi: 10.1073/pnas.81.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Nucleotide sequence of the promoter region of the xylDEGF operon on TOL plasmid of Pseudomonas putida. Gene. 1984 Sep;29(3):323–330. doi: 10.1016/0378-1119(84)90061-1. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Rogers J. E., Jacob A. E., Hedges R. W. Transposition of Pseudomonas toluene-degrading genes and expression in Escherichia coli. Nature. 1978 Jul 13;274(5667):179–180. doi: 10.1038/274179a0. [DOI] [PubMed] [Google Scholar]
- Jeenes D. J., Williams P. A. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J Bacteriol. 1982 Apr;150(1):188–194. doi: 10.1128/jb.150.1.188-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joakim A., Inniss W. E. Reversible filamentous growth in the psychrophile Bacillus psychrophilus. Cryobiology. 1976 Oct;13(5):563–571. doi: 10.1016/0011-2240(76)90149-8. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach P. R., McGregor I., Ward J. M., Broda P. Molecular relationships between pseudomonas INC P-9 degradative plasmids TOL, NAH, and SAL. Plasmid. 1983 Sep;10(2):164–174. doi: 10.1016/0147-619x(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Mayfield C. I., Inniss W. E. A rapid, simple method for staining bacterial flagella. Can J Microbiol. 1977 Sep;23(9):1311–1313. doi: 10.1139/m77-198. [DOI] [PubMed] [Google Scholar]
- ROSENBERG H., ENNOR A. H., MORRISON J. F. The estimation of arginine. Biochem J. 1956 May;63(1):153–159. doi: 10.1042/bj0630153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Szer W. Cell-free protein synthesis at 0 degrees. An activating factor from ribosomes of a psychrophilic microorganism. Biochim Biophys Acta. 1970 Jul 16;213(1):159–170. [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. L., Dunn N. W. Transmissible plasmid coding for the degradation of benzoate and m-toluate in Pseudomonas arvilla mt-2. Genet Res. 1974 Apr;23(2):227–232. doi: 10.1017/s0016672300014853. [DOI] [PubMed] [Google Scholar]