Abstract
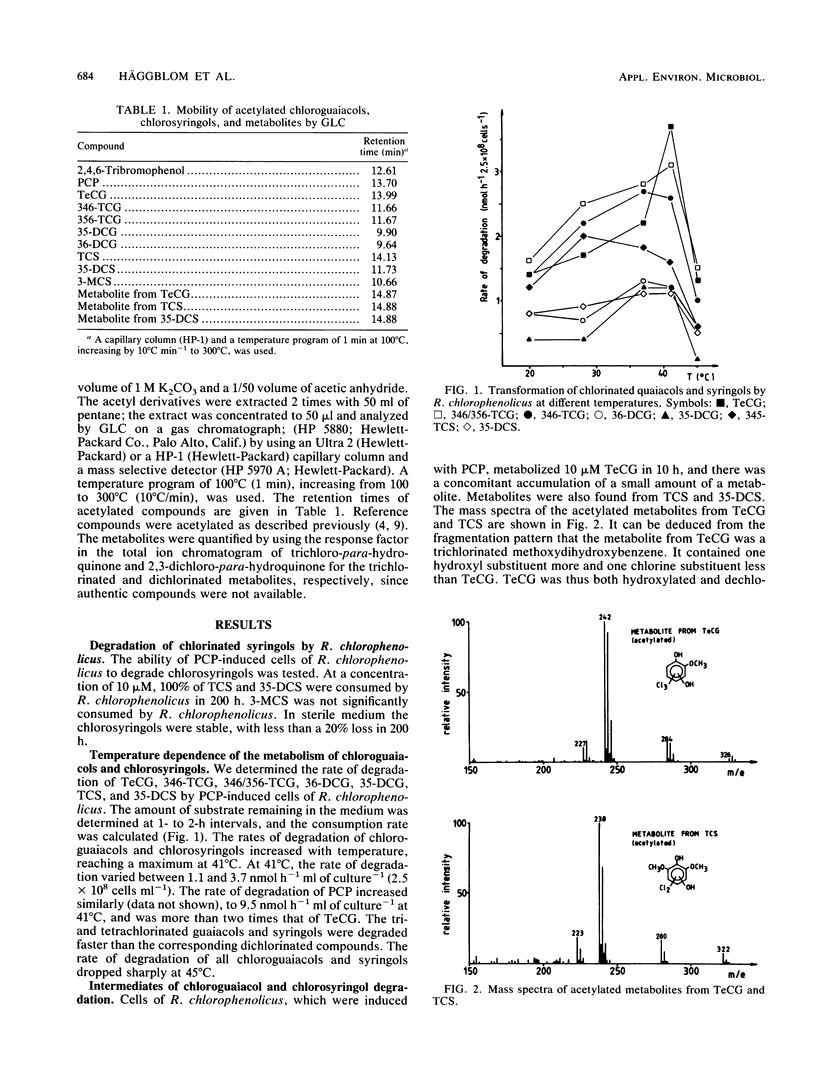
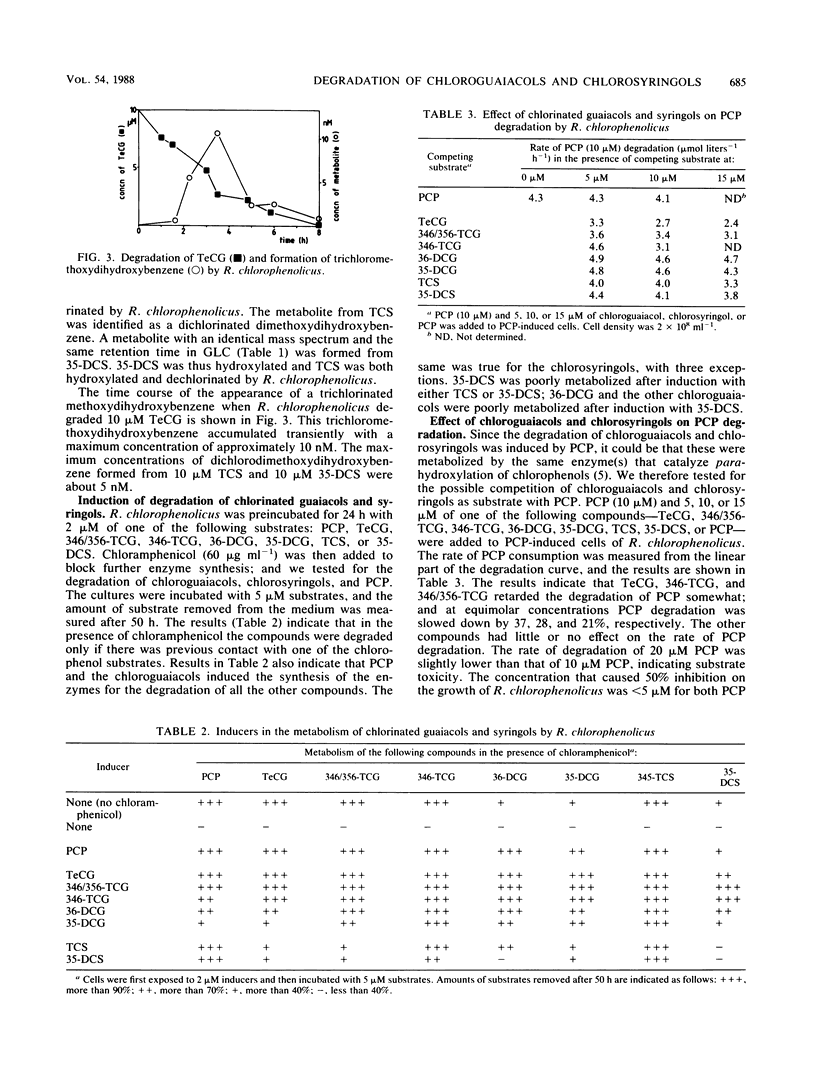
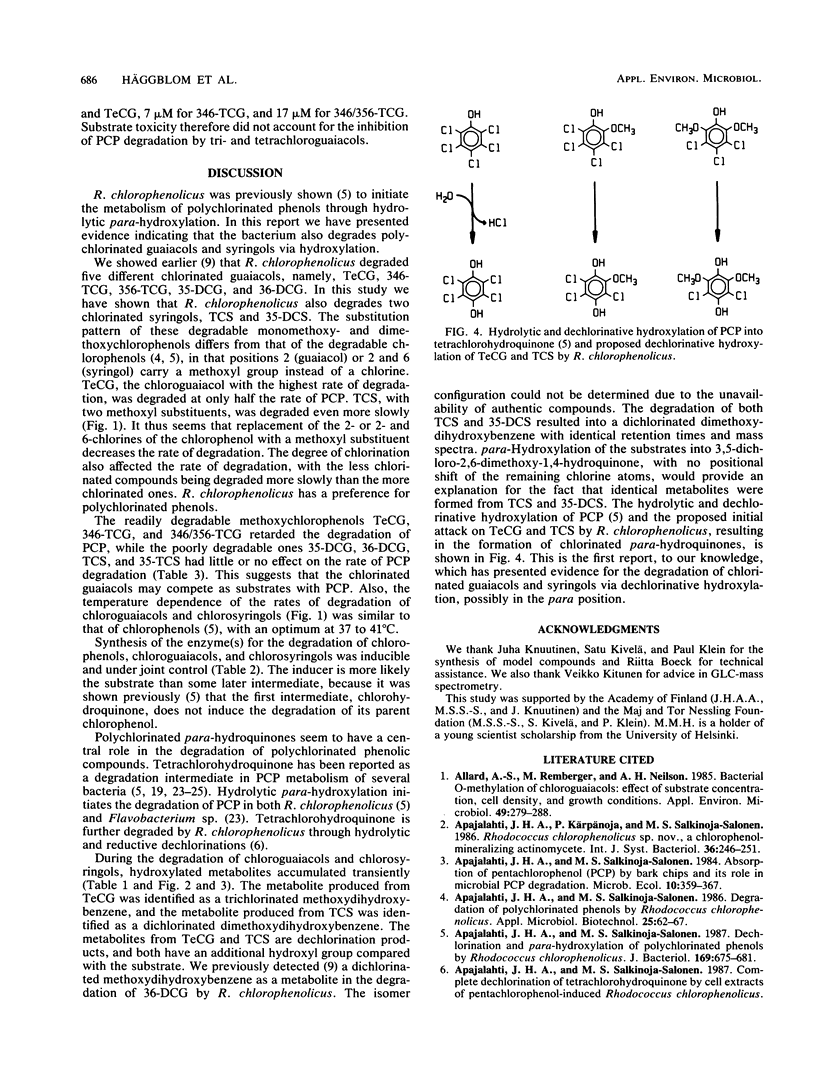
We show that Rhodococcus chlorophenolicus PCP-I, a polychlorophenol degrader, also degrades various chlorine-substituted guaiacols (2-methoxyphenols) and syringols (2,6-dimethoxyphenols). The substrates investigated were tetrachloroguaiacol, 3,4,6- and 3,5,6-trichloroguaiacol, 3,5- and 3,6-dichloroguaiacol, trichlorosyringol, and 3,5-dichlorosyringol. The first step was a hydroxylation, probably in a position para to the preexisting hydroxyl. Tetrachloroguaiacol and trichlorosyringol, with a chlorine substituent in the para position, were both hydroxylated and dechlorinated. The optimum temperature for degradation of polychlorinated guaiacols and syringols was 37 to 41 degrees C. Degradation of polychlorinated phenols, guaiacols, and syringols by R. chlorophenolicus was inducible, and induction was controlled coordinately.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard A. S., Remberger M., Neilson A. H. Bacterial o-methylation of chloroguaiacols: effect of substrate concentration, cell density, and growth conditions. Appl Environ Microbiol. 1985 Feb;49(2):279–288. doi: 10.1128/aem.49.2.279-288.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J. H., Salkinoja-Salonen M. S. Complete dechlorination of tetrachlorohydroquinone by cell extracts of pentachlorophenol-induced Rhodococcus chlorophenolicus. J Bacteriol. 1987 Nov;169(11):5125–5130. doi: 10.1128/jb.169.11.5125-5130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J. H., Salkinoja-Salonen M. S. Dechlorination and para-hydroxylation of polychlorinated phenols by Rhodococcus chlorophenolicus. J Bacteriol. 1987 Feb;169(2):675–681. doi: 10.1128/jb.169.2.675-681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Hynning P. A., Remberger M., Landner L. Bacterial methylation of chlorinated phenols and guaiacols: formation of veratroles from guaiacols and high-molecular-weight chlorinated lignin. Appl Environ Microbiol. 1983 Mar;45(3):774–783. doi: 10.1128/aem.45.3.774-783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Allard A. S., Lindgren C., Remberger M. Transformations of chloroguaiacols, chloroveratroles, and chlorocatechols by stable consortia of anaerobic bacteria. Appl Environ Microbiol. 1987 Oct;53(10):2511–2519. doi: 10.1128/aem.53.10.2511-2519.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remberger M., Allard A. S., Neilson A. H. Biotransformations of chloroguaiacols, chlorocatechols, and chloroveratroles in sediments. Appl Environ Microbiol. 1986 Mar;51(3):552–558. doi: 10.1128/aem.51.3.552-558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiert J. G., Crawford R. L. Catabolism of pentachlorophenol by a Flavobacterium sp. Biochem Biophys Res Commun. 1986 Dec 15;141(2):825–830. doi: 10.1016/s0006-291x(86)80247-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Metabolism of pentachlorophenol by a soil microbe. J Environ Sci Health B. 1977;12(2):113–127. doi: 10.1080/03601237709372057. [DOI] [PubMed] [Google Scholar]



