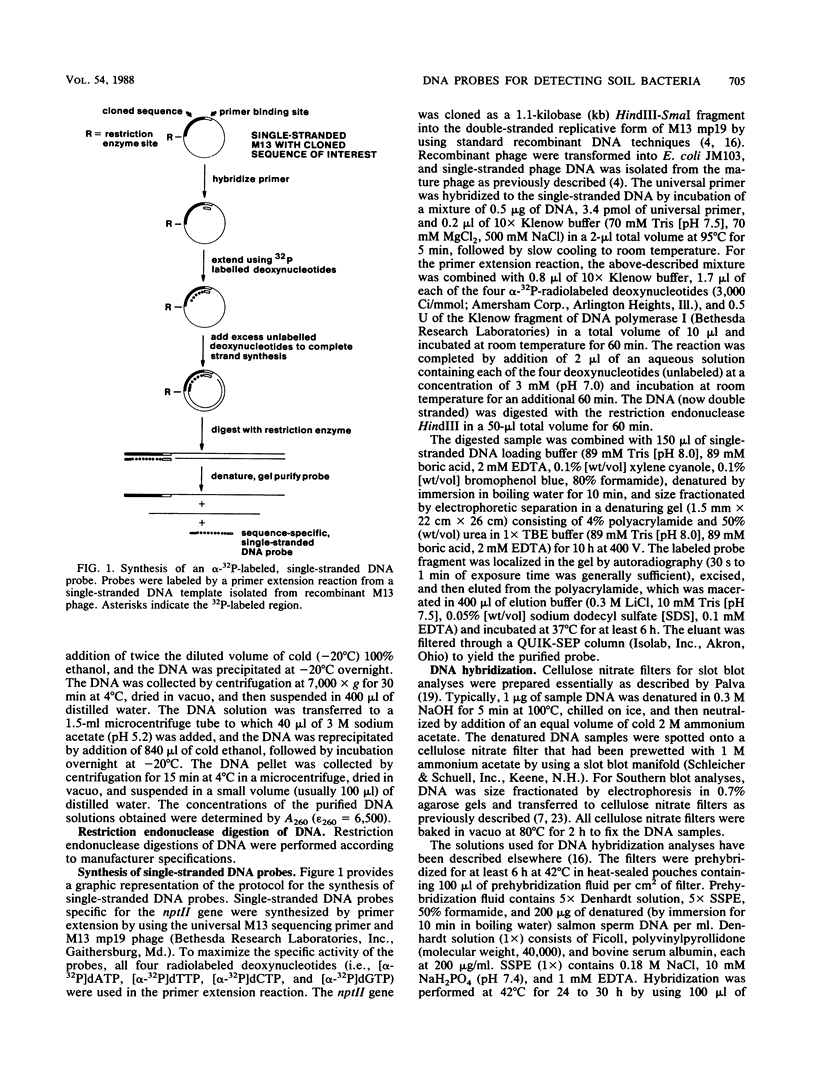
Abstract
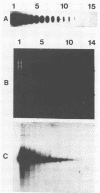
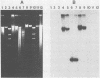
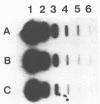
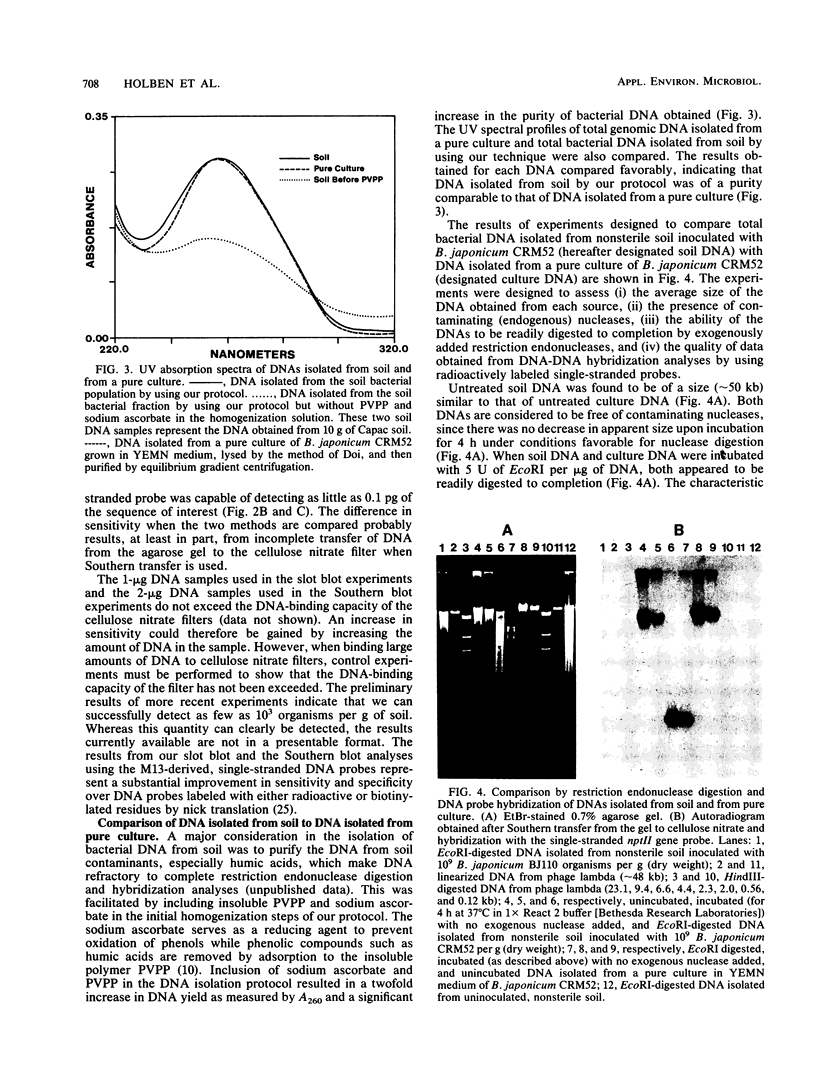
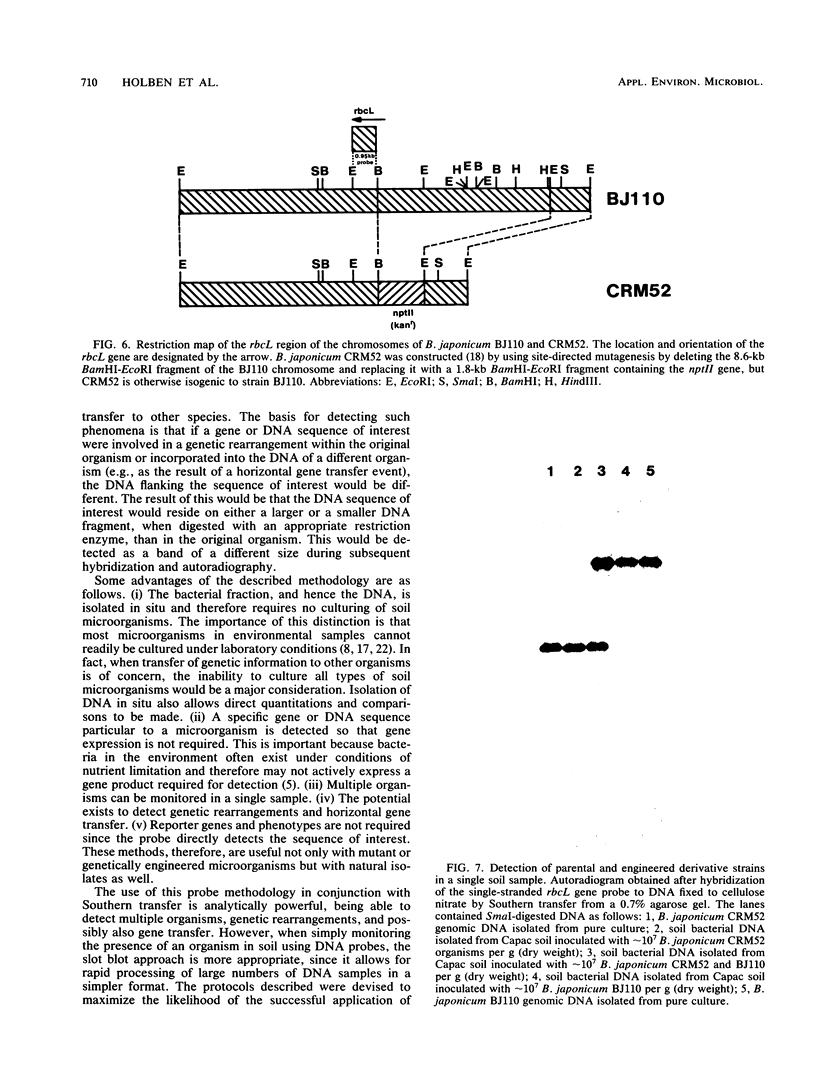
We developed a protocol which yields purified bacterial DNA from the soil bacterial community. The bacteria were first dispersed and separated from soil particles in the presence of polyvinylpolypyrrolidone, which removes humic acid contaminants by adsorption to this insoluble polymer. The soil bacteria were then collected by centrifugation and lysed by using a comprehensive protocol designed to maximize disruption of the various types of bacteria present. Total bacterial DNA was purified from the cell lysate and remaining soil contaminants by using equilibrium density gradients. The isolated DNA was essentially pure as determined by UV spectral analysis, was at least 48 kilobases long, and was not subject to degradation, which indicated that there was no contaminating nuclease activity. The isolated DNA was readily digested by exogenously added restriction endonucleases and successfully analyzed by slot blot and Southern blot hybridizations. Using single-stranded, 32P-labeled DNA probes, we could detect and quantitate the presence of a specific microbial population in the natural soil community on the basis of the presence of a DNA sequence unique to that organism. The sensitivity of our methodology was sufficient to detect Bradyrhizobium japonicum at densities as low as 4.3 × 104 cells per g (dry weight) of soil, which corresponds to about 0.2 pg of hybridizable DNA in a 1-μg DNA sample.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakken L. R. Separation and purification of bacteria from soil. Appl Environ Microbiol. 1985 Jun;49(6):1482–1487. doi: 10.1128/aem.49.6.1482-1487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Labeda D. P., Casida L. E., Jr Simplified procedures for releasing and concentrating microorganisms from soil for transmission electron microscopy viewing as thin-sectioned and frozen-etched preparations. Can J Microbiol. 1975 Mar;21(3):252–262. doi: 10.1139/m75-036. [DOI] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette G. K., Jezeski J. J., McFeters G. A., Stuart D. G. Influence of environmental stress on enumeration of indicator bacteria from natural waters. Appl Microbiol. 1975 Feb;29(2):186–194. doi: 10.1128/am.29.2.186-194.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone T. L., Balkwill D. L. Improved flotation technique for microscopy of in situ soil and sediment microorganisms. Appl Environ Microbiol. 1986 Mar;51(3):462–468. doi: 10.1128/aem.51.3.462-468.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Golub E. I., Low K. B. Unrelated conjugative plasmids have sequences which are homologous to the leading region of the F factor. J Bacteriol. 1986 May;166(2):670–672. doi: 10.1128/jb.166.2.670-672.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. W., Martin W. J. Microwave oven for melting laboratory media. J Clin Microbiol. 1978 Apr;7(4):401–402. doi: 10.1128/jcm.7.4.401-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C. R., Chelm B. K. A genetic locus essential for formate-dependent growth of Bradyrhizobium japonicum. J Bacteriol. 1987 Jul;169(7):3260–3267. doi: 10.1128/jb.169.7.3260-3267.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]