Abstract
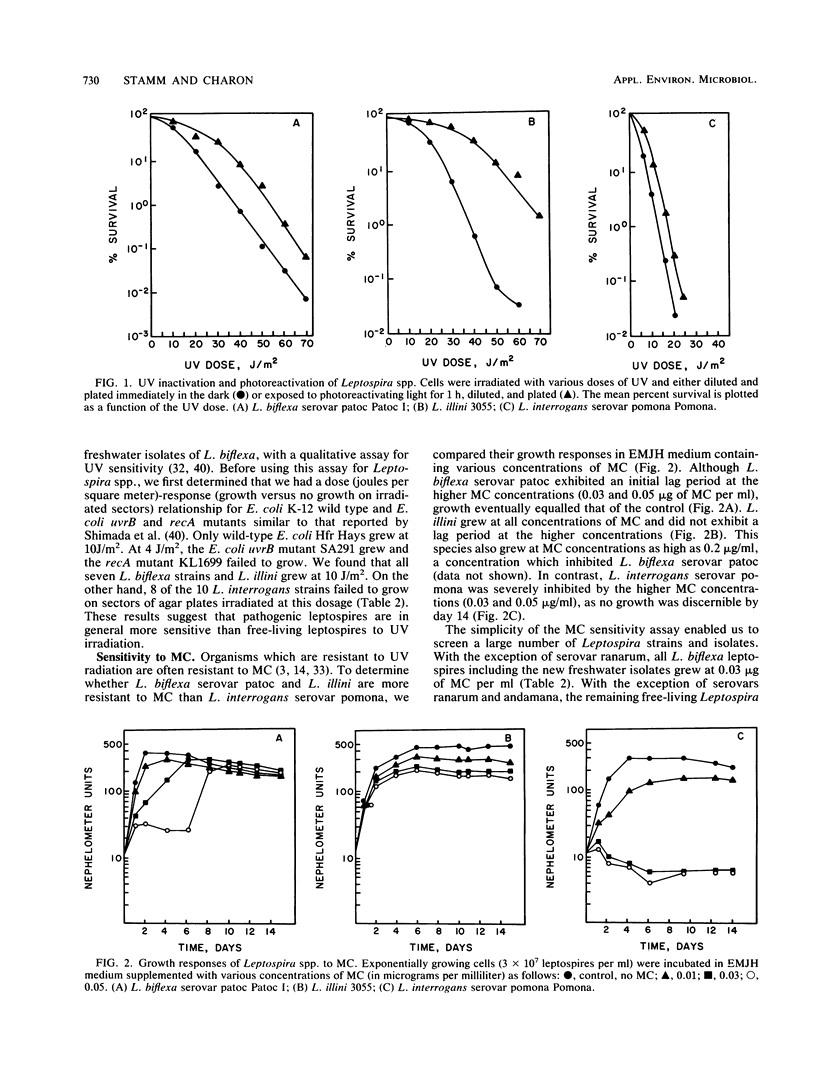
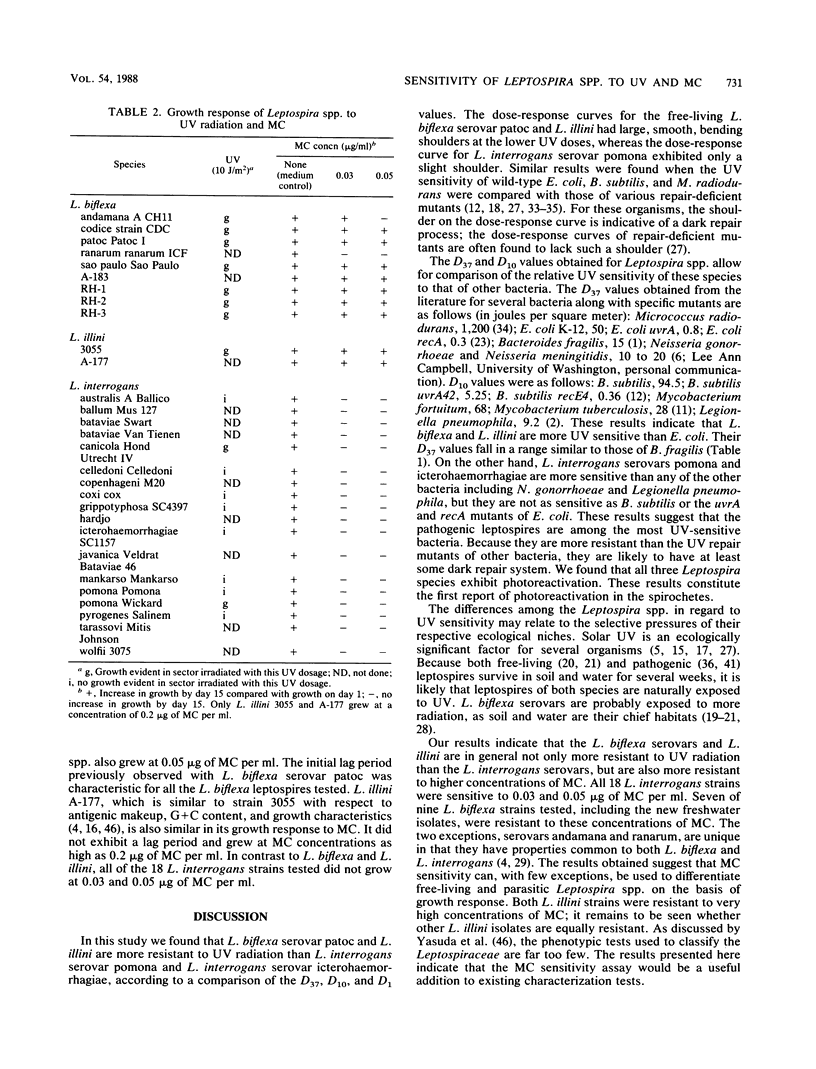
The habitats for the two major Leptospira spp. differ. The main habitat of L. biflexa is soil and water, whereas L. interrogans primarily resides in the renal tubules of animals. We investigated whether these two species, along with L. illini (species incertae sedis), differ with respect to their sensitivity to UV radiation. The doses of UV resulting in 37, 10, and 1% survival were determined for representative serovars from each species. L. interrogans serovar pomona was 3.0 to 4.8 times more sensitive to UV than the other Leptospira species under the 37, 10, and 1% survival parameters. In comparison to other bacteria, L. interrogans serovar pomona is among the most sensitive to UV. In a qualitative UV sensitivity assay, L. interrogans serovars were found to be in general more sensitive than L. biflexa serovars. All three species were found to have a photoreactivation DNA repair mechanism. Since organisms that are resistant to UV are often resistant to the DNA cross-linking agent mitomycin C, we tested the relative sensitivity of several Leptospira serovars to this compound. With few exceptions, L. biflexa and L. illini serovars were considerably more resistant to mitomycin C than the L. interrogans serovars. The mitomycin C sensitivity assay could be a useful addition to current characterization tests used to differentiate the Leptospira species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abratt V. R., Jones D. T., Woods D. R. Isolation and physiological characterization of mitomycin C-sensitive/UV-sensitive mutants in Bacteroides fragilis. J Gen Microbiol. 1985 Sep;131(9):2479–2483. doi: 10.1099/00221287-131-9-2479. [DOI] [PubMed] [Google Scholar]
- Antopol S. C., Ellner P. D. Susceptibility of Legionella pneumophila to ultraviolet radiation. Appl Environ Microbiol. 1979 Aug;38(2):347–348. doi: 10.1128/aem.38.2.347-348.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Yasbin R. E. Deoxyribonucleic acid repair capacities of Neisseria gonorrhoeae: absence of photoreactivation. J Bacteriol. 1979 Dec;140(3):1109–1111. doi: 10.1128/jb.140.3.1109-1111.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Campbell A. M., Stamm S. C. Isolation of lambda transducing phage with the bio genes inserted between lambda genes P and Q. Genetics. 1980 May;95(1):1–13. doi: 10.1093/genetics/95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Johnson R. C., Peterson D. Amino acid biosynthesis in the spirochete Leptospira: evidence for a novel pathway of isoleucine biosynthesis. J Bacteriol. 1974 Jan;117(1):203–211. doi: 10.1128/jb.117.1.203-211.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L. Response of Mycobacteria to ultraviolet light radiation. Am Rev Respir Dis. 1973 Nov;108(5):1175–1185. doi: 10.1164/arrd.1973.108.5.1175. [DOI] [PubMed] [Google Scholar]
- FUZI M., CSOKA R. [Differentiation of pathogenic and saprophytic Leptospira with a copper sulfate test]. Zentralbl Bakteriol. 1960 Jun;179:231–237. [PubMed] [Google Scholar]
- Friedman B. M., Yasbin R. E. The genetics and specificity of the constitutive excision repair system of Bacillus subtilis. Mol Gen Genet. 1983;190(3):481–486. doi: 10.1007/BF00331080. [DOI] [PubMed] [Google Scholar]
- GREENBERG J., MANDELL J. D., WOODY P. L. Resistance and cross-resistance of Escherichia coli mutants to antitumour agent mitomycin C. J Gen Microbiol. 1961 Nov;26:509–520. doi: 10.1099/00221287-26-3-509. [DOI] [PubMed] [Google Scholar]
- Harm W. Biological determination of the germicidal activity of sunlight. Radiat Res. 1969 Oct;40(1):63–69. [PubMed] [Google Scholar]
- Harwood C. S., Canale-Parola E. Ecology of spirochetes. Annu Rev Microbiol. 1984;38:161–192. doi: 10.1146/annurev.mi.38.100184.001113. [DOI] [PubMed] [Google Scholar]
- Henry R. A., Johnson R. C., Bohlool B. B., Schmidt E. L. Detection of Leptospira in soil and water by immunofluorescence staining. Appl Microbiol. 1971 May;21(5):953–956. doi: 10.1128/am.21.5.953-956.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. A., Johnson R. C. Distribution of the genus Leptospira in soil and water. Appl Environ Microbiol. 1978 Mar;35(3):492–499. doi: 10.1128/aem.35.3.492-499.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. MITOMYCINS AND PORFIROMYCIN: CHEMICAL MECHANISM OF ACTIVATION AND CROSS-LINKING OF DNA. Science. 1964 Jul 3;145(3627):55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. DIFFERENTIATION OF PATHOGENIC AND SAPROPHYTIC LEPTOSPIRES WITH 8-AZAGUANINE. J Bacteriol. 1964 Dec;88:1618–1623. doi: 10.1128/jb.88.6.1618-1623.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Purine analogue sensitivity and lipase activity of leptospires. Appl Microbiol. 1968 Oct;16(10):1584–1590. doi: 10.1128/am.16.10.1584-1590.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson G. B. Photoreactivation of ultraviolet-irradiated, plasmid-bearing, and plasmid-free strains of Bacillus anthracis. Appl Environ Microbiol. 1986 Sep;52(3):444–449. doi: 10.1128/aem.52.3.444-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. F. Four mutants of Micrococcus radiodurans defective in the ability to repair DNA damaged by mitomycin-C, two of which have wild-type resistance to ultraviolet radiation. Mol Gen Genet. 1978 Apr 17;160(3):331–337. doi: 10.1007/BF00332977. [DOI] [PubMed] [Google Scholar]
- Moseley B. E. The isolation and some properties of radiation-sensitive mutants of Micrococcus radiodurans. J Gen Microbiol. 1967 Nov;49(2):293–300. doi: 10.1099/00221287-49-2-293. [DOI] [PubMed] [Google Scholar]
- Moss S. H., Davies D. J. Interrelationship of repair mechanisms in ultraviolet-irradiated Escherichia coli. J Bacteriol. 1974 Oct;120(1):15–23. doi: 10.1128/jb.120.1.15-23.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI W., RINGEN L. M. Some effects of various environmental conditions on the survival of Leptospira pomona. Am J Vet Res. 1957 Jan;18(66):219–223. [PubMed] [Google Scholar]
- SMITH D. J., SELF H. R. Observations on the survival of Leptospira australis A in soil and water. J Hyg (Lond) 1955 Dec;53(4):436–444. doi: 10.1017/s0022172400000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Tripathy D. N., Hanson L. E. Studies of Leptospira illini, strain 3055: pathogenicity for different animals. Am J Vet Res. 1973 Apr;34(4):557–562. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]


