Abstract
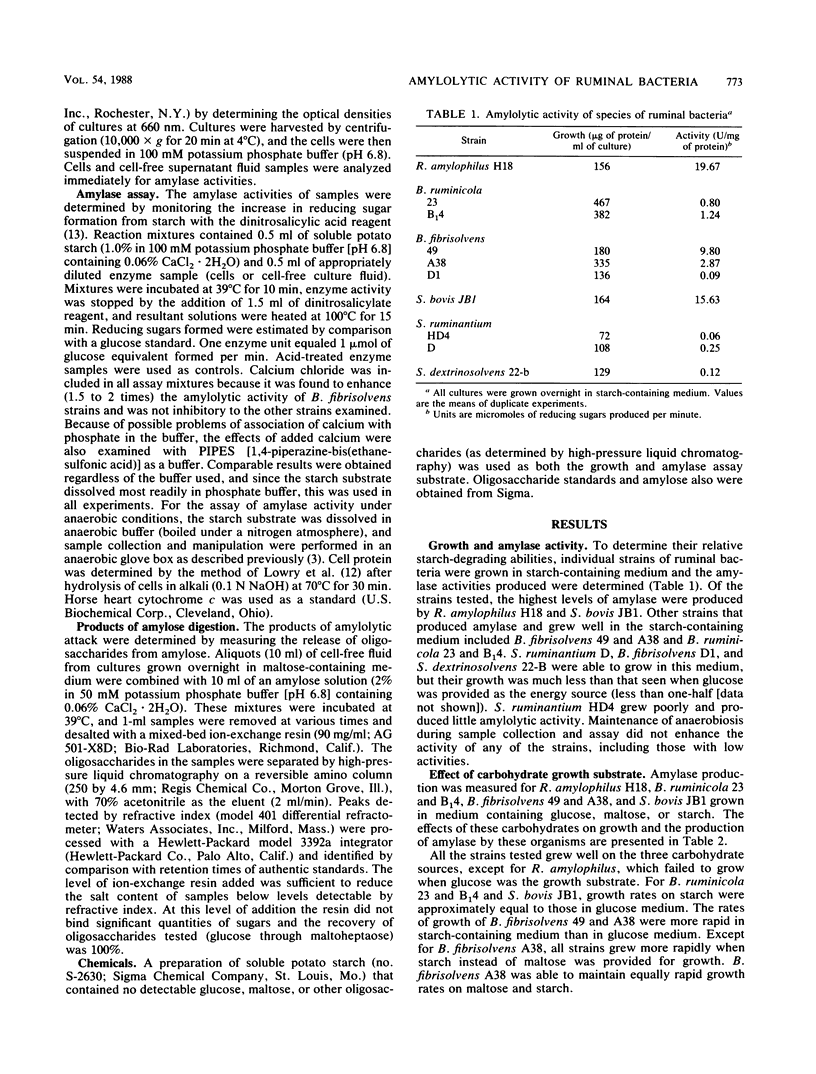
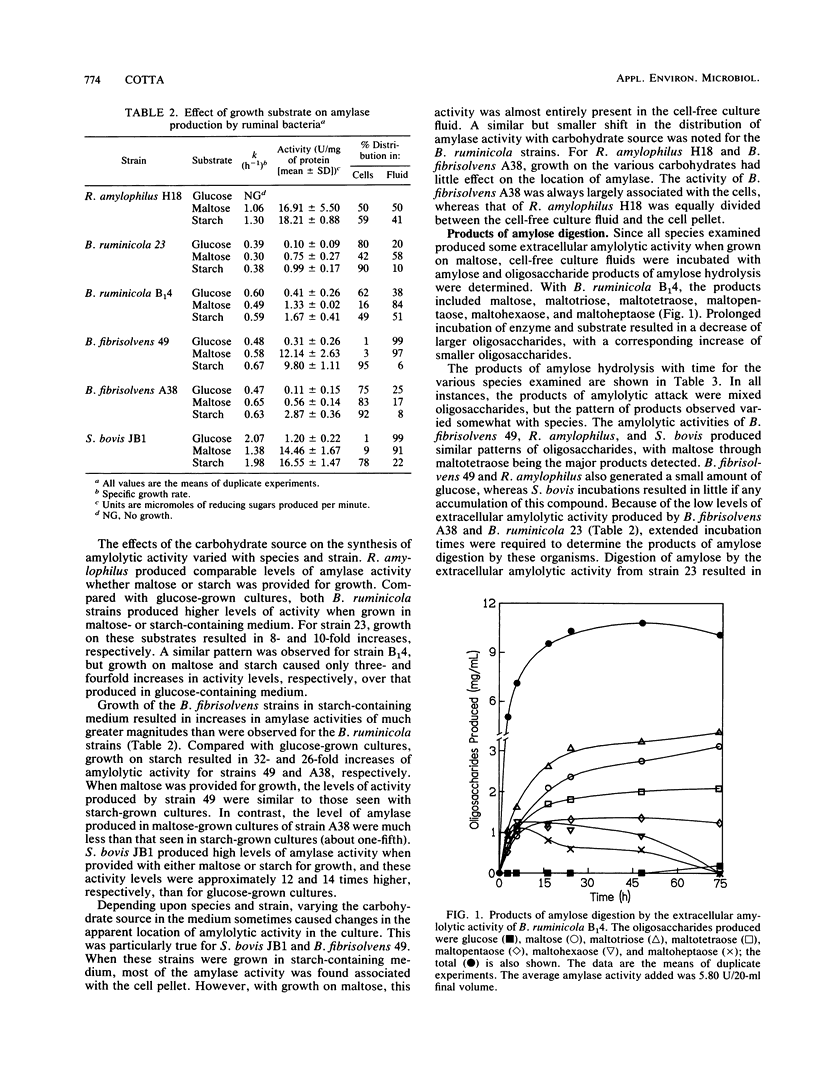
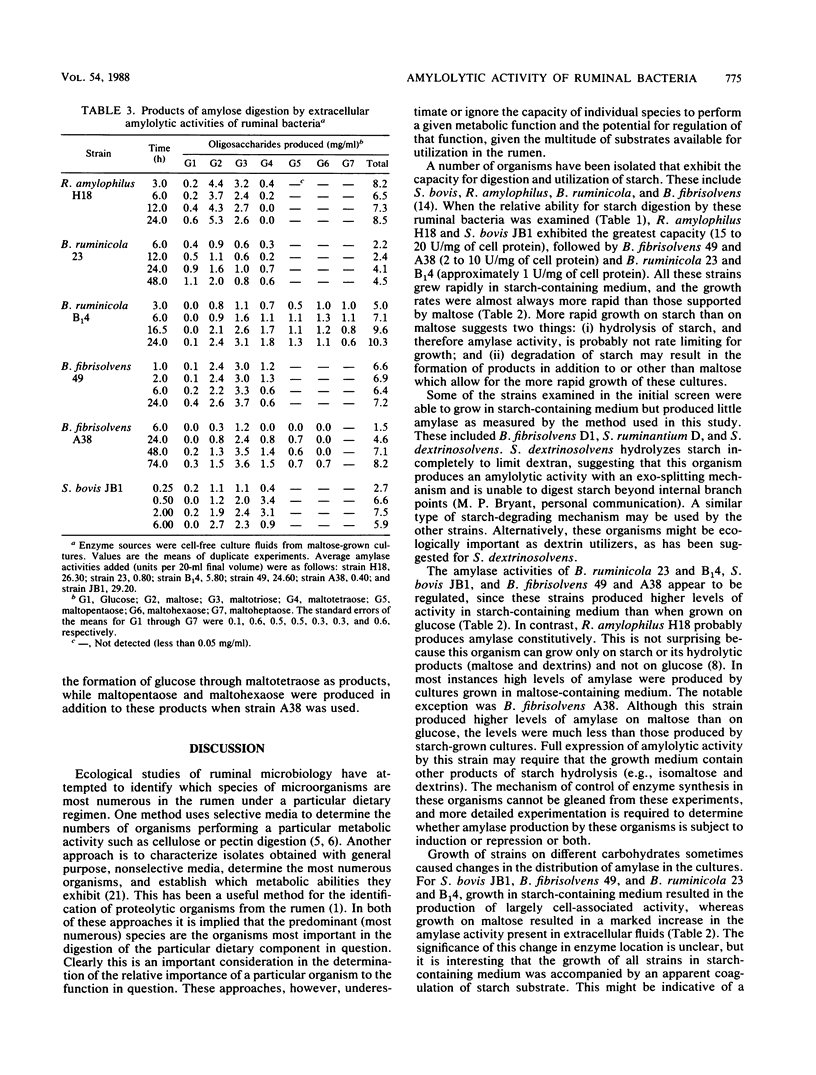
A variety of species of ruminal bacteria were screened for the ability to grow in starch-containing medium and produce amylase. Of those tested, the highest levels of amylase were produced by Streptococcus bovis JB1 and Ruminobacter amylophilus H18. Other strains that grew well on starch and produced amylase included Butyrivibrio fibrisolvens A38 and 49 and Bacteroides ruminicola 23 and B14. Varying the carbohydrate source provided for growth resulted in changes in the growth rate and level of amylase produced by these strains. All strains grew rapidly in starch-containing medium, and the rates of growth were generally more rapid than those observed for maltose-grown cultures. For S. bovis JB1, B. ruminicola 23 and B14, and B. fibrisolvens 49 and A38, amylase was produced when growth was on maltose or starch, but this activity was greatly reduced in glucose-grown cultures. The distribution of amylolytic activity between cellular and extracellular fractions was sometimes affected by the carbohydrate provided for growth. If S. bovis JB1 and B. fibrisolvens 49 were grown on starch, amylase was largely associated with cell pellets; however, if grown on maltose these strains produced activities that were almost entirely present in the extracellular fluid fractions. Although not as dramatic, a similar shift in the location of amylase activities was noted for the two B. ruminicola strains when grown on the same substrates. Growth on maltose or starch had little influence on either the predominantly cell-associated activity of B. fibrisolvens A38 or the activity of R. amylophilus H18, which was equally divided between cell pellet and extracellular fluid fractions.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKBURN T. H., HOBSON P. N. Further studies on the isolation of proteolytic bacteria from the sheep rumen. J Gen Microbiol. 1962 Sep;29:69–81. doi: 10.1099/00221287-29-1-69. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N. Characteristics of two new genera of anaerobic curved rods isolated from the rumen of cattle. J Bacteriol. 1956 Jul;72(1):22–26. doi: 10.1128/jb.72.1.22-26.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A., Hespell R. B. Proteolytic activity of the ruminal bacterium Butyrivibrio fibrisolvens. Appl Environ Microbiol. 1986 Jul;52(1):51–58. doi: 10.1128/aem.52.1.51-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Pectin-fermenting bacteria isolated from the bovine rumen. J Bacteriol. 1969 Jul;99(1):189–196. doi: 10.1128/jb.99.1.189-196.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMLIN L. J., HUNGATE R. E. Culture and physiology of a starch-digesting bacterium (Bacteroides amylophilus n. sp.) from the bovine rumen. J Bacteriol. 1956 Oct;72(4):548–554. doi: 10.1128/jb.72.4.548-554.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N., MACPHERSON M. Amylases of Clostridium butyricum and a Streptococcus isolated from the rumen of the sheep. Biochem J. 1952 Dec;52(4):671–679. doi: 10.1042/bj0520671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E., DOUGHERTY R. W., BRYANT M. P., CELLO R. M. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 1952 Oct;42(4):423–449. [PubMed] [Google Scholar]
- Hespell R. B., Wolf R., Bothast R. J. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2849–2853. doi: 10.1128/aem.53.12.2849-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Russell J. B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985 Mar;49(3):572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Theurer C. B. Grain processing effects on starch utilization by ruminants. J Anim Sci. 1986 Nov;63(5):1649–1662. doi: 10.2527/jas1986.6351649x. [DOI] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Hope P. M. Degradation of starch granules by some amylolytic bacteria from the rumen of sheep. Biochem J. 1964 Feb;90(2):398–408. doi: 10.1042/bj0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]


