Abstract
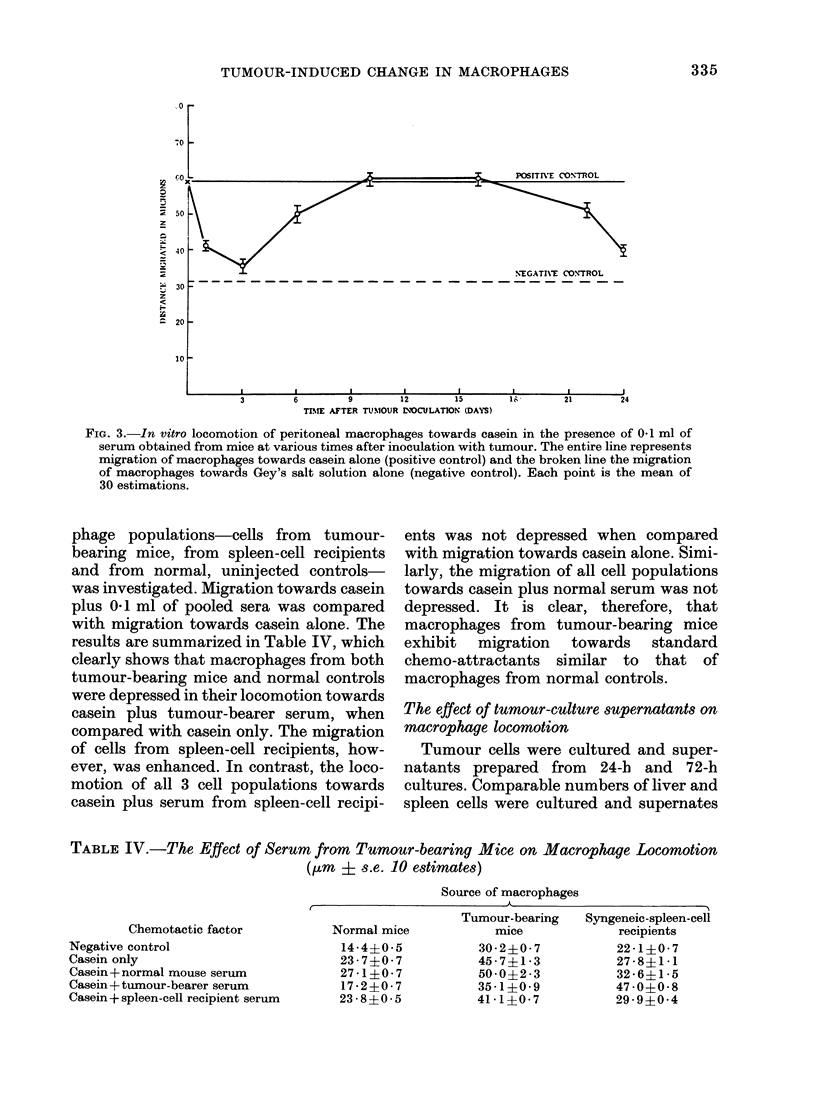
The function of the reticulo-endothelial system in mice bearing Lewis lung carcinomas has been measured by(1) the rate of clearance of carbon particles from the circulation in vivo and calculation of the phagocytic index K; (2) chemotactic locomotion of macrophages in vitro in the presence or absence of serum or tumour supernate. The ability of the bone marrow to develop macrophage colonies in vitro in the presence or absence of sera from tumour-bearing mice has also been tested. A clear depression of macrophage locomotion and macrophage colony formation in vitro was found in the presence of sera or tumour supernates from tumour-bearing mice as early as 24 to 72 h after tumour inoculation. Similarly, tumour-bearing mice showed marked depression of carbon clearance in tests repeated throughout the first 72 h after tumour inoculation. This early depression of macrophage function may be an important step in allowing escape of tumour cells from host resistance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., STIFFEL C., HALPERN B. N., MOUTON D. Etude de la fonction phagocytaire du S.R.E. au cours du développment de tumeurs malignes expérimentales chez le rat et la souris. Ann Inst Pasteur (Paris) 1958 Jun;94(6):681–693. [PubMed] [Google Scholar]
- Baum M., Breese M. Antitumour effect of corynebacterium parvum. Possible mode of action. Br J Cancer. 1976 Apr;33(4):468–473. doi: 10.1038/bjc.1976.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M., Fisher B. Macrophage production by the bone marrow of tumor-bearing mice. Cancer Res. 1972 Dec;32(12):2813–2817. [PubMed] [Google Scholar]
- Blamey R. W. Experiments in tumour immunology. Br J Surg. 1968 Oct;55(10):769–771. doi: 10.1002/bjs.1800551013. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Evans R., Salaman M. H. Studies on the mechanism of action of Riley virus. 3. Replication of Riley's plasma enzyme-elevating virus in vitro. J Exp Med. 1965 Nov 1;122(5):993–1002. doi: 10.1084/jem.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groch G. S., Perillie P. E., Finch S. C. Reticuloendothelial phagocytic function in patients with leukemia, lymphoma and multiple myeloma. Blood. 1965 Oct;26(4):489–499. [PubMed] [Google Scholar]
- Kampschmidt R. F., Pulliam L. A. Changes in the opsonin and cellular influences on phagocytosis during the growth of transplantable tumors. J Reticuloendothel Soc. 1972 Jan;11(1):1–10. [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. I. Continuous cluster formation and relation of clusters to colonies. J Cell Physiol. 1969 Dec;74(3):323–332. doi: 10.1002/jcp.1040740313. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. II. Action of colony stimulating factor. J Cell Physiol. 1970 Aug;76(1):89–99. doi: 10.1002/jcp.1040760113. [DOI] [PubMed] [Google Scholar]
- OLD L. J., BENACERRAF B., CLARKE D. A., CARSWELL E. A., STOCKERT E. The role of the reticuloendothelial system in the host reaction to neoplasia. Cancer Res. 1961 Oct;21:1281–1300. [PubMed] [Google Scholar]
- Otu A. A., Russell R. J., White R. G. Biphasic pattern of activation of the reticuloendothelial system by anaerobic coryneforms in mice. Immunology. 1977 Mar;32(3):255–264. [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Antikatzides T. G. Humoral mediated macrophage response during tumour growth. Br J Cancer. 1975 Oct;32(4):471–482. doi: 10.1038/bjc.1975.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsbury A. J., Burrage K., Hellmann K. Inhibition of metastatic spread by I.C.R.F. 159: selective deletion of a malignant characteristic. Br Med J. 1970 Nov 7;4(5731):344–346. doi: 10.1136/bmj.4.5731.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Blaylock B. L., Weinstein P. Effects of neoplasms on inflammation: depression of macrophage accumulation after tumor implantation. J Immunol. 1976 Mar;116(3):585–589. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



