Abstract
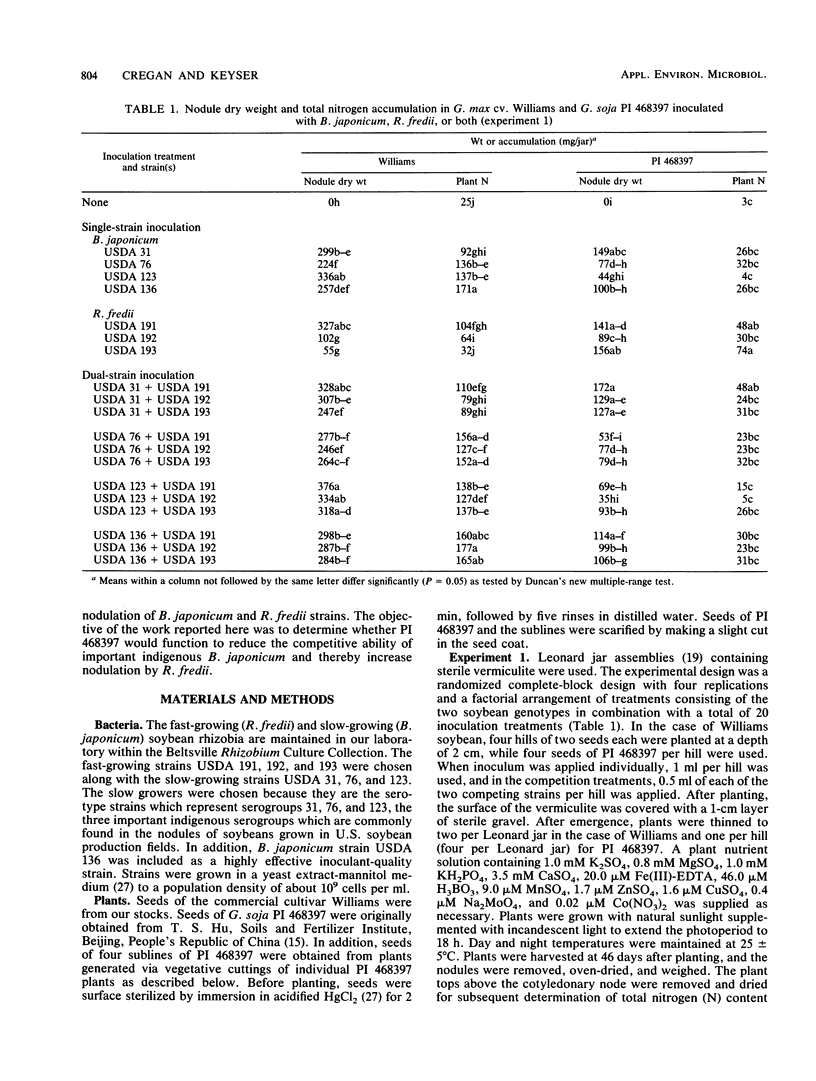
The displacement of indigenous Bradyrhizobium japonicum in soybean nodules with more effective strains offers the possibility of enhanced N2 fixation in soybean (Glycine max (L.) Merr.). Our objective was to determine whether the wild soybean (G. soja Sieb. & Zucc.) genotype PI 468397 would cause reduced competitiveness of important indigenous B. japonicum strains USDA 31, 76, and 123 and thereby permit nodulation by Rhizobium fredii, the fast-growing microsymbiont of soybean. In an initial experiment, PI 468397 nodulated and fixed moderate amounts of N2 with USDA 31 and 76 but, despite the formation of nodules, fixed essentially no N2 with USDA 123. In contrast, PI 468397 formed a highly effective symbiosis with R. fredii strain USDA 193. In two subsequent experiments, Williams soybean and PI 468397 were grown in a pasteurized soil mixture or in soybean rhizobium-free soil and inoculated with both USDA 123 and USDA 193. In each experiment, more than 90% of the nodules of Williams contained USDA 123, while only a maximum of 2% were occupied with USDA 193. In contrast, in the two experiments, 16 and 11%, respectively, of the nodules produced on PI 468397 were occupied by USDA 123, while in both experiments 87% contained USDA 193. Thus, in relation to the cultivar Williams, which is commonly grown and used as a parent in soybean breeding programs in the United States, PI 468397 substantially reduced the competitive ability of B. japonicum strain USDA 123 in relation to R. fredii strain USDA 193.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dowdle S. F., Bohlool B. B. Predominance of Fast-Growing Rhizobium japonicum in a Soybean Field in the People's Republic of China. Appl Environ Microbiol. 1985 Nov;50(5):1171–1176. doi: 10.1128/aem.50.5.1171-1176.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Mixed inoculations with effective and ineffective strains of Rhizobium leguminosarum. J Appl Bacteriol. 1976 Jun;40(3):375–380. doi: 10.1111/j.1365-2672.1976.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Kuykendall L. D., Weber D. F. Genetically marked Rhizobium identifiable as inoculum strain in nodules of soybean plants grown in fields populated with Rhizobium japonicum. Appl Environ Microbiol. 1978 Dec;36(6):915–919. doi: 10.1128/aem.36.6.915-919.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L. T. A Simple Assembly for Use in the Testing of Cultures of Rhizobia. J Bacteriol. 1943 Jun;45(6):523–527. doi: 10.1128/jb.45.6.523-527.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mytton L. R. Plant genotype times rhizobium strain interactions in white clover. Ann Appl Biol. 1975 May;80(1):103–107. doi: 10.1111/j.1744-7348.1975.tb01604.x. [DOI] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


