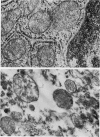
Abstract
Mitochondrial fractions isolated from tumours induced with the respiratory inhibitor rotenone lack respiratory control, oxidative phosphorylation, are partially or totally insensitive to cyanide and have a near-normal content of respiratory carriers. These characteristics are more similar to those of mitochondria from atrophic mammary gland than to those of mitochondria from spontaneous mammary adenomas. Thus, the characteristic structural and biochemical mitochondrial alteration of rotenone-induced tumours would represent a lack of mitochondrial differentiation as the tumour develops from the atrophic mammary gland. Slices of rotenone-induced tumours are insensitive to oligomycin and dinitrophenol, thus indicating that glycolysis would be their sole source of metabolic energy.
Full text
PDF

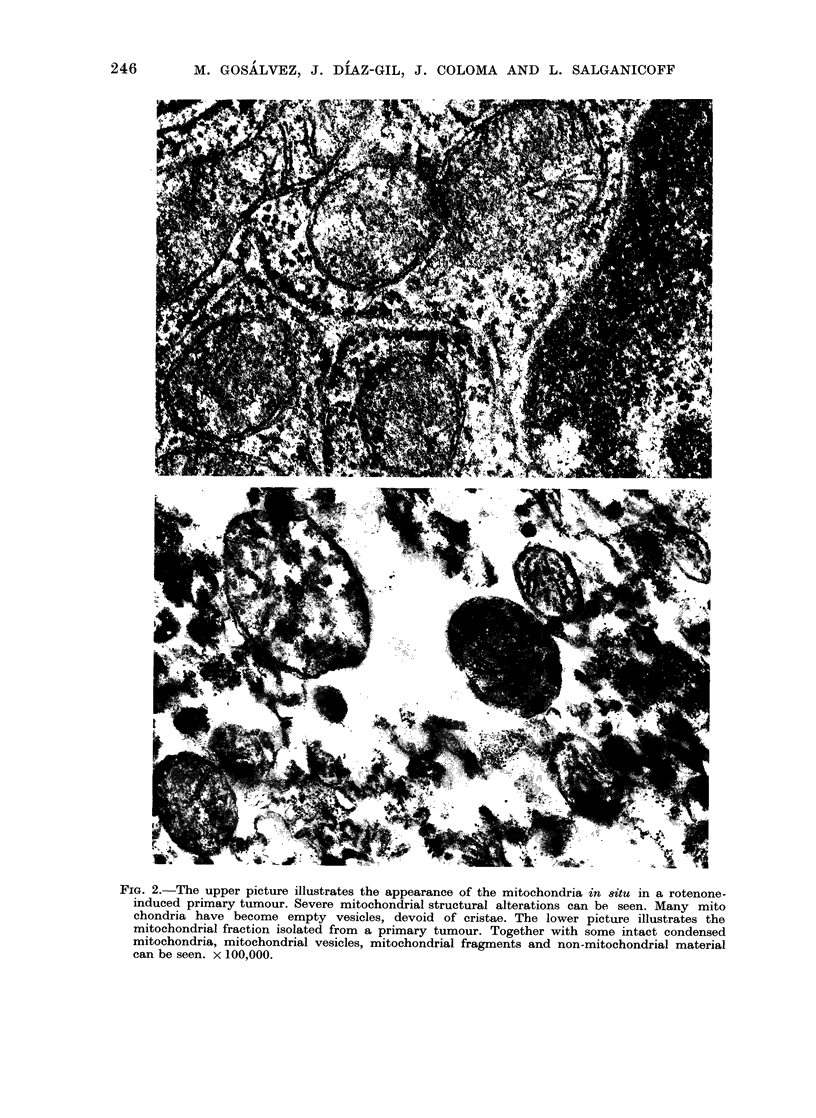
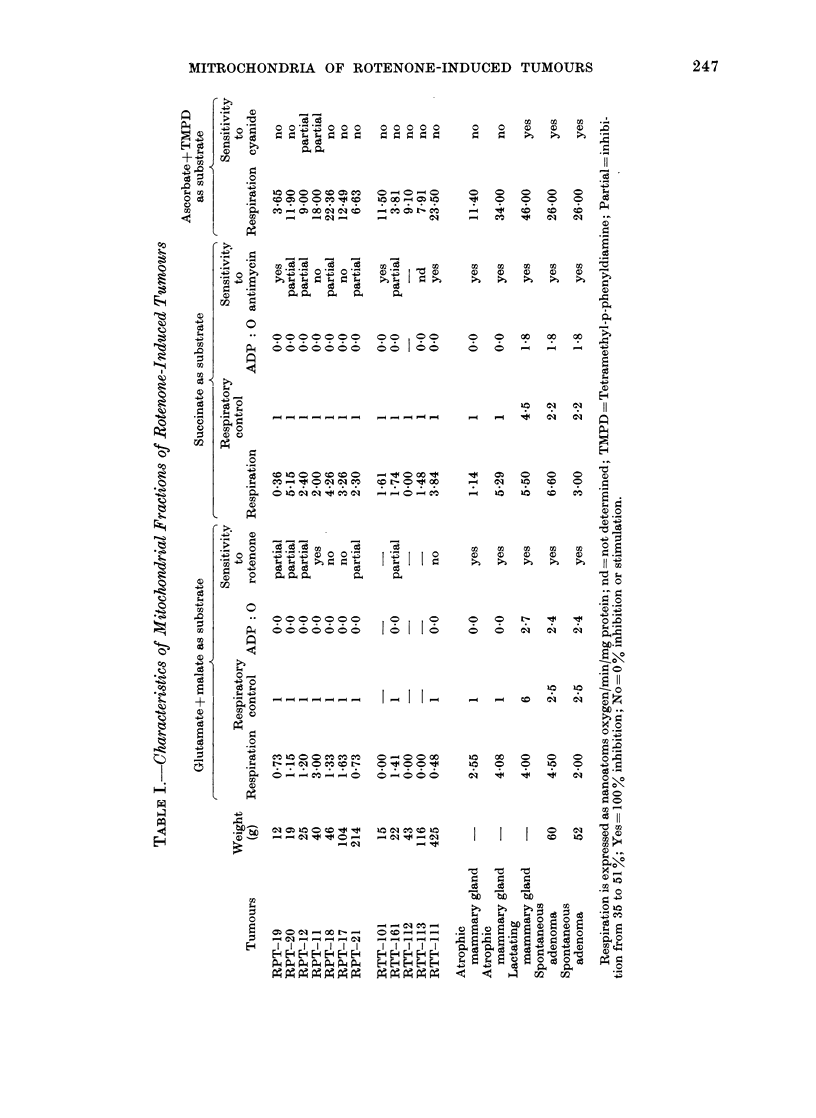


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. The kinetics and inhibition of cytochrome components of the succinic oxidase system. I. Activity determinations and purity criteria. J Biol Chem. 1952 May;197(2):557–565. [PubMed] [Google Scholar]
- CHANCE B. The kinetics and inhibition of cytochrome components of the succinic oxidase system. III. Cytochrome b. J Biol Chem. 1958 Nov;233(5):1223–1229. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R., HOLLUNGER G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem. 1963 Jan;238:418–431. [PubMed] [Google Scholar]
- CHAPPELL J. B., PERRY S. V. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954 Jun 5;173(4414):1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W. Kinetic properties of a reduced diphosphopyridine nucleotide cytochrome c reductase from heart muscle. J Biol Chem. 1957 Aug;227(2):1093–1108. [PubMed] [Google Scholar]
- Gosalvez M., García-Cañero R., Reinhold H. Delayed pyridine nucleotide reoxidation induced by the anticancer agent VM-26 as measured in vivo and in situ by NADH microfluorimetry. Eur J Cancer. 1975 Oct;11(10):709–715. doi: 10.1016/0014-2964(75)90044-4. [DOI] [PubMed] [Google Scholar]
- Gosalvez M., López-Alarcón L., García-Suarez S., Montalvo A., Weinhouse S. Stimulation of tumor-cell respiration by inhibitors of pyruvate kinase. Eur J Biochem. 1975 Jun 16;55(1):315–321. doi: 10.1111/j.1432-1033.1975.tb02165.x. [DOI] [PubMed] [Google Scholar]
- Gosalvez M., Merchan J. Letter: Induction of rat mammary adenomas with respiratory inhibitory rotenone. Cancer Res. 1973 Nov;33(11):3047–3050. [PubMed] [Google Scholar]
- Gosalvez M., Pérez-García J., Weinhouse S. Competition for ADP between pyruvate kinase and mitochondrial oxidative phosphorylation as a control mechanism in glycolysis. Eur J Biochem. 1974 Jul 1;46(1):133–140. doi: 10.1111/j.1432-1033.1974.tb03605.x. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., JURTSHUK P., HAAVIK A. G. Studies on the electron transport system. XXXI. DPNH-cytochrome c reductase II. Biochim Biophys Acta. 1961 Sep 2;52:119–129. doi: 10.1016/0006-3002(61)90909-x. [DOI] [PubMed] [Google Scholar]
- Hanes D. M., Tappel A. L. Lysosomal hemochromes and digestion of cytochrome c by the lysosomal protease system. Biochim Biophys Acta. 1971 Aug 6;245(1):42–53. doi: 10.1016/0005-2728(71)90006-5. [DOI] [PubMed] [Google Scholar]
- Juretic D. Cyanide-resistant respiration of a Neurospora crassa membrane mutant. J Bacteriol. 1976 Apr;126(1):542–543. doi: 10.1128/jb.126.1.542-543.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENBERG M. Pigments of rat liver microsomes. Arch Biochem Biophys. 1958 Jun;75(2):376–386. doi: 10.1016/0003-9861(58)90436-3. [DOI] [PubMed] [Google Scholar]
- Mehard C. W., Packer L., Abraham S. Activity and ultrastructure of mitochondria from mouse mammary gland and mammary adenocarcinoma. Cancer Res. 1971 Dec;31(12):2148–2160. [PubMed] [Google Scholar]
- Negelein E., Leistner I., Jähnchen L. Anwendung der Manometrie zur Untersuchung des Zellwachstums beim Ehrlich-Asziteskarzinom. Einfluss der Sauerstoffkonzentration auf die Zellvermehrung. Acta Biol Med Ger. 1966;16(4):372–387. [PubMed] [Google Scholar]
- Palmer G., Horgan D. J., Tisdale H., Singer T. P., Beinert H. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XIV. Location of the sites of inhibition of rotenone, barbiturates, and piericidin by means of electron paramagnetic resonance spectroscopy. J Biol Chem. 1968 Feb 25;243(4):844–847. [PubMed] [Google Scholar]
- Sato N., Hagihara B. Spectrophotometric analyses of cytochromes in ascites hepatomas of rats and mice. Cancer Res. 1970 Jul;30(7):2061–2068. [PubMed] [Google Scholar]
- Storey B. T. Effect of cyanide and Antimycin A on the reduction of cytochrome b and ubiquinone in electron transport particles. Arch Biochem Biophys. 1967 Aug;121(2):271–278. doi: 10.1016/0003-9861(67)90076-8. [DOI] [PubMed] [Google Scholar]
- Van Rossum G. D., Gosalvez M., Galeotti T., Morris H. P. Net movements of monovalent and bivalent cations, and their relation to energy metabolism, in slices of hepatoma 3924A and of a mammary tumour. Biochim Biophys Acta. 1971 Sep 7;245(2):263–276. doi: 10.1016/0005-2728(71)90145-9. [DOI] [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C. Cytochrome P-450cam. II. Interconversion with P-420. J Biol Chem. 1974 Jan 10;249(1):102–106. [PubMed] [Google Scholar]